Most Water Distribution Companies used the standard of 6.5 to 8.5 in treatment facilities but once it leaves the distribution system too much pH(Alkaline) or too little pH(Acidic) can affect the corrosive nature of the water by the time it enters your home. What is the safe pH level of Drinking Water?
EPA recommends but doesn’t require drinking water pH should be between 6.5 to 8.5 on a scale ranging from 0 to 14. The best pH level for drinking water should sit right in the middle of 7.0. but runs a little higher in the distribution system at a range outside this recommendation may be a sign that the H2O contains heavy metals, minerals, or chemicals.
Considerable advancement and knowledge of corrosion control science and interactions with other water treatment objectives are pH-dependent. Once the treated water leaves the plant is where pH needs to be stable to keep your water healthy until it reaches your Tap.
Safe pH Level for Drinking Water
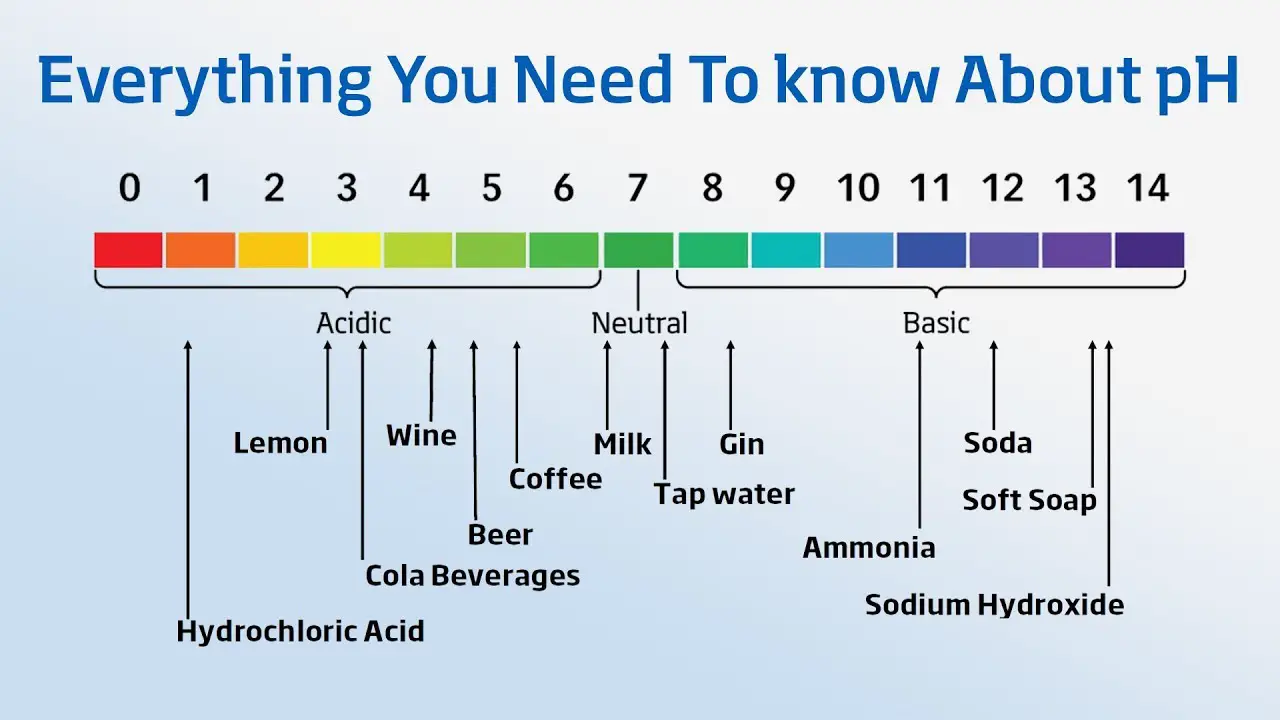
The pH level of your drinking water shows how acidic it is. pH stands for “potential of hydrogen,” referring to the amount of hydrogen found in a substance which in this case, is water. pH is measured on a scale that runs from 0 to 14. Seven is neutral, meaning that represents the balance between acid and alkalinity. A measurement below 7 means acid is present and a measurement above 7 is basic or it is alkaline.
Water that contains more free hydrogen ions is acidic, and water that contains more free hydroxyl ions is basic. Since pH can be affected by chemicals in the water, pH is an important indicator of water that is changing chemically. pH is reported in “logarithmic units”. Each number represents a 10-fold change in the acidity/basic of the water. Water with a pH of five is ten times more acidic than water having a pH of six.
The EPA or the (Environmental Protection Agency) does not recognize pH as a Primary regulation or parameter in Drinking water. It is recognized as a Secondary Contaminate whose impact is considered aesthetic. Still, the EPA recommends that public water systems maintain pH levels of between 6.5 and 8.5, being a good guide for individual private well owners.
How Does pH Affect Drinking Water
Water with a low or acidic range can be corrosive. Drinking water that is lower in pH can leach metals from pipes and fixtures, such as copper, lead, and zinc. It can damage metal pipes and affect the taste of drinking water. It also will over time contain diluted medals that end up at your faucet. It can lower the effects of disinfection making it necessary to add more.
At the other end of the pH Scale, High pH or Alkaline drinking water can contain high levels of minerals that pose no health threat but can damage electric water heaters and develop scale and staining that damages plumbing and the taste of potable water.
In Water Treatment just as in Wastewater treatment, most processes in order to work well are pH-dependent. So before a chemical is added to a treatment plant the pH needs to be adjusted so that the chemicals can perform well. In Water Treatment facilities coagulation, precipitation, disinfection, and corrosion control are dependent on pH, These types of treatment help clean your water so it leaves the plant ready to drink.
These steps in treatment are compartmentalized each performing its function. The next step is dependent on the previous step for successful treatment. The steps of treatment need a balanced pH to work. It works much the same way you’re swimming pool is dependent on a balanced pH for disinfection. In Treatment facilities, online pH monitoring is used and alarmed to keep this process control optimized at a high level. Your Water Treatment Facility does a great job of keeping this standard, operating in a specific tight range that is maintained continuously.
There is a worry. Aging Infrastructures that have been deteriorating over the last 100 years are between the proper treatment that Municipalities and Cities all through our Country deliver. Water Treatment Facilities are the most examined and looked at Industries, held to standards that are tested and monitored constantly. The miles of piping that delivers the drinking water to you in most cases are not.
The current pH range should be re-examined in light of the new science to ensure that water quality is optimized. In the distribution system, buffering capacity (maintaining a stable pH) is at its minimum in the approximate range of pH 8.3-8.5, and considerable improvements are seen in practice at higher pH.
How to Test pH Level In Water
Testing for the pH of your Drinking Water is warranted and the easiest easy way to check and ensure the water you drink is healthy for you and your family. Not only does the pH of a receiving stream affect organisms living in the water outside, but a changing pH in a stream can also be an indicator of increasing pollution. This works similarly to Drinking Water leaving the treatment plant with a pH that is normally able to buffer any changes that may happen during its time in the distribution system.
The pH of water can determine its solubility. In the case of heavy metals, the degree to which they are soluble determines their toxicity. Metals tend to be more toxic at lower pH because they are more soluble. Contact your state or local health department for a list of certified laboratories that can test the pH level of your water. If your water is acidic less than 7 pH you may have problems with the leaching of copper and lead from your plumbing. Consider testing for copper and lead if the pH test shows your water is highly acidic.
If testing indicates that your water has a high pH, consider testing for alkalinity and hardness as well, as these can be associated with high pH water. I did this Calibration video on my new Digital pH meter in order to set it up to measure a drinking water difference between my raw faucet City Water and after being treated by a Berkey Gravity Filter. That result is in a later video.
- Sample your faucet at the first draw or immediately after you turn it on
- Run your faucet from your main source for 30 seconds or so and take the second sample.
- Before you take a sample for pH make sure to calibrate your digital pH meter.
- Keep the sample in glass or plastic containers and collect enough samples to be able to merge and cover the probes on the meter.
- Adjust for temperature or let the sample come to room temperature.
- Don’t shake or agitate the sample while reading but gently stir the sample until the meter starts to stabilize.
What Causes Galvanic Corrosion
The Science of Drinking Water Corrosion is a series of reactions between the water and metal surfaces and materials where the water is stored or transported. The corrosion process is an oxidation/reduction reaction that returns refined or processed metal to its more stable ore state adding its compounds to the drinking water.
corrosion process is an oxidation/reduction reaction that returns refined or processed metal to its more stable ore state adding its compounds to the drinking water.
If you are experiencing blue/green staining in the sinks and/or bathtubs or pinholes forming in your copper pipes, this can be a sign of corrosion. A low pH is another. The primary concerns are Lead and then Copper which is diluted in the drinking water.
Copper and lead can be toxic and can possibly leach into tap water in older or new homes. This leaching is caused by pipe corrosion. Copper contamination can cause gastrointestinal problems in the short term and damage the liver and kidneys over time. It’s extremely toxic Lead contamination can cause physical and mental development problems in children. In adults, it can lead to high blood pressure and kidney problems. Because of the severity of Lead poisoning in drinking water, EPA banned the use of Lead soldering in 1986.
The primary source of copper is the leaching of copper from the household piping used to convey the water throughout the home. In some cases, the water is so corrosive that the interior plumbing system needs to be changed and completely replaced with PVC piping, PEX (which carries a huge expense), or other similar materials. Other metals that are leached from the distribution of drinking water include chromium, copper, lead, and zinc.
 The following are the recommended maximum contaminant levels for regulated public water supplies for the metals: chromium (0.05 ppm), copper (1 ppm), lead (0.05 ppm), and zinc (5 ppm). To protect the public, the EPA requires public water supplies to be non-corrosive and the “Lead and Copper Rule” has set new action levels for lead and copper of 0.015 ppm and 1.3 ppm, respectively. Because of the toxicity of lead to children, the EPA has established a recommended maximum contaminant level of 0 ppm for lead.
The following are the recommended maximum contaminant levels for regulated public water supplies for the metals: chromium (0.05 ppm), copper (1 ppm), lead (0.05 ppm), and zinc (5 ppm). To protect the public, the EPA requires public water supplies to be non-corrosive and the “Lead and Copper Rule” has set new action levels for lead and copper of 0.015 ppm and 1.3 ppm, respectively. Because of the toxicity of lead to children, the EPA has established a recommended maximum contaminant level of 0 ppm for lead.
Corrosion will occur anywhere a galvanic cell or field can be or has been established. This happens when 2 dissimilar metals are connected directly or indirectly by an electrolyte which is water in the pipe. This is the same reaction that occurs in a battery.
Minerals dissolved in water separate into charged particles (ions) that conduct electricity. Conductivity is a problem only when the water has a high mineral content; pure water does not conduct electricity. Corrosive water coming from a Public or Private Well systems cause household plumbing and metal fixtures to resulting in the deterioration of the pipes and increased metal content of the water.
The worst-case scenario can cause elevated toxic levels in your drinking water. If any of these metals or contaminants are of worry to you then you really can’t depend on a pH test. The same result that leads to the Disaster in Flint Michigan can happen anywhere where the Infrastructure is aging and deteriorating faster than it can be replaced. That’s pretty much everywhere in the Nation.
Average pH of Well Water
There are over 13 million private wells used by citizens in the Country. EPA does not regulate private wells nor does it provide recommended criteria or standards for individual wells. You should test your Private Well once a year for total coliform bacteria, nitrates, total dissolved solids, pH levels, or anything you suspect. Or provide a sample for the Health Department to test by a certified Lab.
Shallow Wells as opposed to deep Wells contain more Dissolved Oxygen and have higher temperatures than deep Wells which can help cause corrosion in pipes. High dissolved solids, from salts and sulfates, sodium, chloride, or other ions found in Well water also help to contribute to the problem. Oxygen and dissolved CO2 or other gasses can induce corrosion The pH reading is an important indicator and a drastic change could indicate existing problems and a few areas of contamination such as:
- Household plumbing or service lines that contain lead
- Corrosion of pipes, plumbing
- Coal or other mining operations nearby
- Dump, junkyard, landfill, factory, gas station, or dry-cleaning operation nearby
EPA Secondary Drinking Water Standards
High and Low pH can help contribute to problems with aesthetic effects (such as taste, odor, or color) in drinking water. Water pipes and water-using appliances can become encrusted with deposits and can depress the effectiveness of disinfection by chlorine, thereby causing the need for additional chlorine when pH is high.
Low-pH water will corrode or dissolve metals and other substances. Stain sinks, cause pitting on water pipes and containers that hold water. These are subtle changes that get worse dissolving heavy metals like Lead and Copper can have adverse effects on human health.
EPA’s (NSDWR) or the National Secondary Drinking Water Regulations are non-enforceable guidelines regulating contaminants that may cause cosmetic effects such as skin or tooth discoloration or aesthetic effects (such as taste, odor, or color) in drinking water.
EPA recommends secondary standards for water systems but does not require systems to comply with the standard. However, states may choose to adopt them as enforceable standards. The safe range for leaving the Water Treatment facility is still 6.5-8.5 pH until further studies on the Science of Corrosion in the future, experimenting with minimum pH levels of 8.2-8.5 in the Drinking Water Distribution grid to protect against corrosion of old piping that leads the result of dangerous contaminants involved in the Drinking Water.
JimGalloway/Author/Editor
Lead In Drinking Water – Is There Lead In My City Drinking Water?
Drinking Water Regulations and Contaminants