When you add Chlorine to your pool water it forms hypochlorous acid. How effective it works as a disinfectant is dictated and influenced by the other chemicals that exist in the pool, primarily pH and the number of bathers, and the waste they leave behind. What is Combined Chlorine?
Combined Chlorine is the portion of chlorine in the water that has reacted and combined with ammonia, such as nitrogen-containing contaminants, and other organics that come from a swimmer’s perspiration, urine, and other waste.
Some posts I see on this subject make it out to be “Rocket Science” It’s Not! Combined chlorine levels are routinely monitored alongside residual chlorine, and are a necessary guide to the health of pool water. To be a good pool operator it’s sometimes necessary to play detective using the knowledge you gained from experience.
Combined Chlorine
Combined chlorine refers to the mixture of chlorine with organic amines and ammonia which forms when free chlorine(FC) combines with other chemicals and water pollutants involved in the pool water and ultimately loses its disinfectant properties.
Combined chlorine in pool water and spas forms when free chlorine has combined with other chemicals in the water and in this state is unable to sanitize or purify the water. Free chlorine molecule has combined with water pollutants to form combined chlorine, ultimately losing its disinfectant properties.
When chlorine is added to water, it first forms hypochlorous acid (HOCl) and hypochlorite ions (OCl-). Hypochlorous acid is a strong bacterial-killing form of chlorine while hypochlorite ion is a weaker form of chlorine in the water. When substances such as ammonia are available they mix and form inorganic chloramines, they react with both forms (HOCl) & (OCl-) of chlorine to generate Combined chlorine.
Combined Chlorine Levels
The combined chlorine residual result should ideally be zero, or no more than half the free chlorine residual, and even if the free chlorine is higher and over 2mg/l then it still should be less than 1mg/l. If the combined chlorine is at or above 1mg/l, then further testing and investigation should be done to determine the source of the problem.
Combined chlorine is the chlorine that has already been used after you add it to sanitize your water. When chlorine in the pool water comes in contact with organic material, such as skin oils, urine, or sweat, they react to form combined chlorine, also known as chloramines.
Combined chlorine is known as “bad chlorine” which causes the smell, red eyes, and irritated skin that’s a complaint from many pool users. If Combined Chlorine starts to move over o.2 ppm then chloramines will start to accumulate and should be considered a problem and investigated before getting any higher.
Combined Chlorine Too High
There are a few solutions to high or climbing Combined Chlorine readings but the easiest is to add more shock chlorine. If maintaining the correct level of chlorination reduces combined chlorine, the pool water is satisfactory. If increasing free chlorine also increases the combined chlorine, the pool water is unsatisfactory. Adding shock chlorine until the Combined residual falls off and the Free Chlorine residual shows up is called breakpoint chlorination.
Make sure you use a reliable kit with fresh reagents(check the expiration dates on the bottles) before Testing Pool Water.
If you smell chlorine, coming off the surface of the water, what you really smell are combined forms of chlorine, also called chloramines. Chloramines are chemical compounds formed by chlorine combined with nitrogen-containing contaminants in the pool water.
These, are still disinfectants, but they are 40 to 60 times less effective than free available chlorine. Contaminates come from swimmer wastes such as sweat, urine, body oil, etc. This is why requiring all bathers to take a warm, soapy water shower is a good idea.
Three types of chloramines can be formed in water mono-chloramine, dichloramine, and trichloramine.
- Monochloramine is formed from the reaction of hypochlorous acid with ammonia.
- Monochloramine may then react with more hypochlorous acid to form a Dichloramine.
- Dichloramine may react with hypochlorous acid to form a trichloramine.
Trichloramines cause the “chlorine” smell and hang in the air directly above the pool water level, often causing frequent swimmers to have unhealthy asthma-like symptoms. Always use a reliable color comparator pool tester with fresh reagents and get a fairly accurate result of Combined Chlorine. If you don’t have one then buy one and don’t forget to add fresh reagents every season.
Breakpoint Chlorination
- The equation will be Combined Chlorine-Total Chlorine=Free Chlorine (FC)
- If the water has no Chloramines then the answer will equal= 0 (no shock treatment is needed-this its the optimum level)
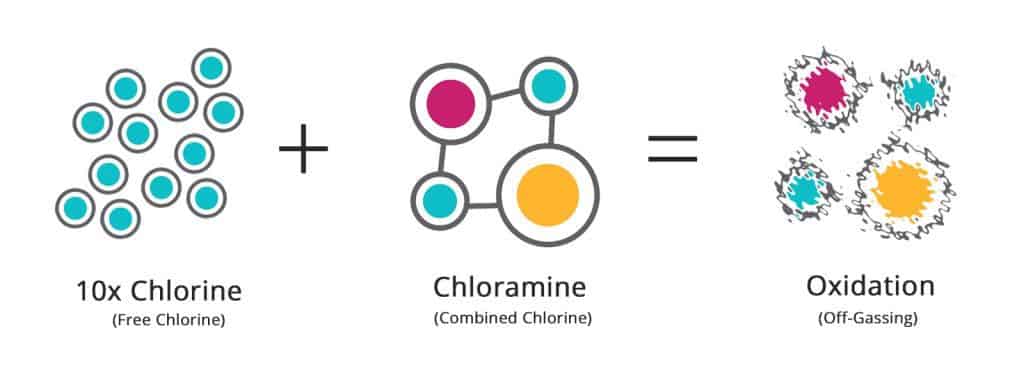
- The breakpoint chlorination value is 10 times the combined chlorine (CC) level.
For example 0.8 ppm (CC) from the above example × 10 = 8 ppm of chlorine to achieve breakpoint.
Taking into account the free chlorine already in the pool, the chlorine will have to be added to the level of 8
ppm.

Chloramines can usually be eliminated from the pool H2O by using breakpoint chlorination with chlorine or super oxidation with nonchlorine oxidization. It takes a ratio of chlorine to ammonia atoms of 7.6 to 1 to reach a breakpoint, other contaminants (i.e. bacteria, algae) are also present that must be oxidized, so 10 times the amount of combined chlorine must be added. This should bring your pool back to Free Chlorine (FC) levels.
When sufficient free chlorine is added to pool water, the inorganic chloramines are converted to dichloramine, then to nitrogen trichloride, and then to nitrogen gas where it will leave the pool. Any excess chlorine leftover will become the chlorine residual
Breakpoint Chlorination is one reason why I recommend using Granular Chlorine for disinfecting your pool and spa. You have better control by adding different amounts according to a load of bather activity or other variables like weather control that Chlorine Tablets don’t give you.
What is the Difference Between Free Chlorine and Total Chlorine?
Free chlorine involves the amount of chlorine that’s available to sanitize contaminants while total chlorine is the sum of free chlorine & combined chlorine which is a weaker type of disinfectant directly bound with the .………………………………………………………read more
JimGalloway Author/Editor

Reference: CorrosionPedia- Breakpoint Chlorination
Pool Water Treatment Advisory Group–Combined Chlorine Understanding it and Getting Rid of It
Feature Image Combined Chlorine by Poolonomics.com