Pool owners should remember that when super chlorinating or shocking your swimming pool, chlorine in a granular or liquid form can mess with pH levels at first. Still, if you run a tight ship and have a well-balanced pool, your pool’s water chemistry can handle any changes that come your way and any negative effects that come with using Chlorine. Does Chlorine Affect pH?
Yes-Sodium Hypochlorite or Bleach is Chlorine in liquid form, at 11-13 pH, may change the pH of your pool but won’t last just as Calcium Hypochlorite (“cal hypo”) in granular form when mixed with H2O, may cause an acidic pH change that is also temporary If Total Alkalinity (TA) is between 80-90 ppm.
Pool water starts with a strong chemical structure that will prevent negative happening or fast changes during those dog days of summer. Remember TA or Total Alkalinity is the measurement of your pool’s strong chemical structure which can dictate pH movement that helps convert your chlorine into sanitizing agent once it enters the water in pools.
Does Chlorine Affect pH
Pool owners all know, or at least should know, that Chlorine works the best at the right pH levels, but sometimes, when adding chlorine-like granular or shock chlorine, the pH is affected by the chlorine itself as well as its total alkalinity. When alkalinity falls, it is more difficult to maintain a stable pH which can snowball and lead to problems.
The same process happens in Saltwater Pools where chlorine generators can change the pH in your swimming pool or hot tub. This boost in alkalinity and pH helps balance and then convert your chlorine into a sanitizing agent once it enters the pool water.
Adding Chlorine From a Chlorine Generator Can Affect Pool pH
Higher chlorine levels lower the pH of your pool’s water, making it more acidic, and dropping the levels below 7.0 pH. The more acidic the water, the lower the pH and the higher the likelihood of corrosion. Corrosion can affect metal piping, equipment, and the surface of your pool tiles and concrete. It can also damage pool accessories, ladders liners, and anything else that goes into fresh or saltwater swimming pools.
A normal pH range is considered anything between 7.2 and 7.6, 7.4 being ideal. But it’s not always easy to keep it there. An occasional spike in the pH balance that may happen during super chlorinating your pool isn’t too much to be concerned about, but if you’re constantly battling to keep it in range after you shock your pool, you could have a bigger problem.
Shocking your pool is important, but if you’re using Cal-Hypo (Calcium Hypochlorite), it is one of the various types of chlorine you can buy, and it looks exactly how you’d expect pool chlorine to look. It’s most commonly sold as a white, chlorine-smelling powder/granules, which are poured directly into the pool water or sometimes via the pool skimmer.
It can raise your pool’s pH levels temporarily. The main product is used in shocking your pool, but wait to test your chemistry balance levels consistently, especially after shocking.
Allow the filtration system pumps to circulate the chlorine or any dry chemical to mix and dissolve thoroughly throughout the pool water. It should be well-distributed within a few hours for better results. The best results are after a short time for ensuring a proper pH after the initial spike that usually will happen when you shock swimming pools.
Is Pool Chlorine Acidic or Alkaline
Chlorine pH is neither acidic nor basic because it contains no H+ ions, but when it reacts with water (H2O), forming Hydrochloric and Hypochlorous acids, it becomes an acid solution.
When chlorine gas reacts with water, it forms Hypochlorous acid and Hydrochloric acid, similar to how carbon dioxide reacts to form Carbonic acid. Hypochlorous Acid is ideal when used as a swimming pool disinfectant and sanitizer but creates some acidic properties to the mix. As a result, there is a net increase in the protons in the solution, lowering pH. In Chemistry, it looks like this:
Hypochlorous Acid – is a weak acid with oxidizing properties formed when chlorine dissolves in cold water and is used in bleaching and water treatment.
Hydrochloric Acid-Hydrochloric acid (HCl) is a strong acid. Its pH can be as acidic as low as pH 0.0
So when chlorine shock, either powder or granular, is added to the water (H2O) in your pool, their reaction with water makes forms of acid that can lower the pH in the water’s balance.
Does Adding Liquid Chlorine Affect Pool pH
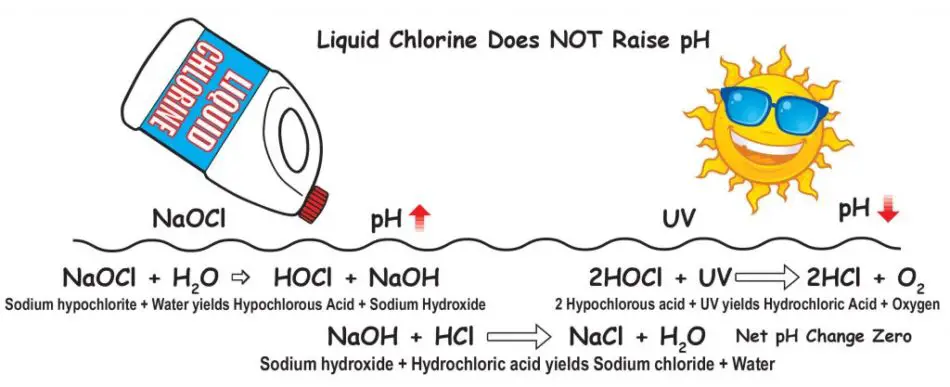
Liquid chlorine and bleach, which are called Sodium hypochlorite or Bleach, have a pH of 11.0 to nearly 13.0, so it is logical to think that they will raise the pH of the pool water. The fact is that initially or upon addition, liquid chlorine raises pH because sodium hydroxide (lye) is made, which is on the base side of the pH scale.
Here is what happens when liquid chlorine sodium hypochlorite – NaOCl is added to pool water:
NaOCl + H2O → HOCl + Na+ + OH
Sodium hypochlorite and water form Hypochlorous acid and Sodium-ion and Hydroxide ion. Remember that hypochlorous acid is HOCl is the killing form of chlorine is made, and sodium ions (Na+) and hydroxide (OH–) are made. The hydroxide raises the pH of the pool water. Liquid chlorine does not raise pH of pool water.
As the liquid chlorine level or Sodium Hypochlorite is consumed in the pool, that small expected spike is reduced with sunlight (UV Light) and agitation to do nothing in a normal size pool; as long as the Total Alkalinity is stable, then this is the strength of balance that will keep low or high spikes from affecting the pH of your swimming pool.
Liquid chlorine does not raise pH. When added to water, liquid chlorine (which has a pH of 13) makes HOCl (hypochlorous acid – the killing form of chlorine) and NaOH (sodium hydroxide), which raises pH. But when the HOCl is degraded by UV, and when used in killing and oxidation, it creates HCl (hydrochloric acid). The amount of HCl is almost identical to the amount of NaOH. So the net effect on pH is zero (or almost zero).
*This is another reason that pool water is so dependent on Total Alkalinity. Most large pool owners agree that keeping a well-balanced pool TA (Total Alkalinity) needs to be in a solid 90-100 ppm range. Start with a Target TA of 90 ppm and adjust up or down in 10 ppm increments until pH remains stable depending on whether the pH is drifting up or down when you’re Testing Pool Water.
Does Cyanuric Acid in Pool Chlorine Affect pH
Cyanuric acid is technically an ‘acid’ but is dissimilar to muriatic acid, which the pool industry uses to manage and adjust pool pH levels. It is sold as a standalone product or can be purchased as a convenient additive to chlorine tablets. It can dissolve in water and has little overall effect on pH, alkalinity, or hardness.
Organic chlorine’s Trichlorisocyanuric Acid and Dichlorisocyanuric Acid dissolve rapidly and is suitable for small pools. Inorganic chlorine is Calcium Hypochlorite for daily and shock chlorination. Sodium Hypochlorite in liquid form has a minimal effect on a swimming pool’s pH level in a well-balanced pool.
Start your pool with a target TA (Total Alkalinity) of 90 ppm and adjust up or down in 10 ppm increments until pH remains stable depending on whether the pH is drifting up or down and help keep these controls these other pool water conditions.
| Free chlorine: Combined chlorine: Total chlorine: Cyanuric acid: Salinity: |
0,5-1,6 ppm (mg/l) 0-0,4 ppm (mg/l) Max 2,0 ppm (mg/l) 30-50 ppm (mg/l) Max 250 ppm (mg/l) (0,025%) |
*Make sure you check Cyanuric acid readings-CYA protects free chlorine from UV destruction, but it also is part of the pH buffering system that keeps the pH from being lowered. CYA also controls how much free chlorine is available for killing and oxidizing.
*If you are using Trichlor as a disinfectant be aware that Trichlor is 55 percent CYA. Using it increases CYA, which requires a higher level of chlorine to kill bacteria and algae. Using trichlor raises CYA by 6 ppm for each 10 ppm of FC. And because it is acidic, it lowers pH and alkalinity.
MyWaterEarth&Sky recommends keeping a schedule for testing your pool’s water. Check this LaMotte Test Kit available through Amazon & get one for the summer. I think using Reagents is the way to go LaMotte ColorQ Pro 9 Plus Digital Liquid Pool & Spa Chemical Water Testing Kit.
Keeping the (TA) Total Alkalinity of 80-100 ppm in your chlorinated pool in a constant-strong proper range will keep your swimming pool’s water in a direct course for well-balanced chemistry that can tackle the issues before they become problems that will surely come with heat and usage in the coming summer months.
For great articles that can keep you out of the Pool Supply Store stay here at MyWaterEarth&Sky for the information, you need to “be the Master of your pool”- pools & spas need reliable testing and sampling procedures using reagents, test strips, or digital probes to perform basic maintenance tests for pH, alkalinity, calcium hardness, and of course chlorine ……………………… Continue reading

References: pH and Chlorine values for good water quality
The Chlorine Institute-Sodium Hypochlorite