All fish and other animals that live in water, like insects, snails, crayfish even microorganisms, require oxygen to survive. Most aquatic animals require certain concentrations of Oxygen to thrive, and depending on how much Dissolved Oxygen is available can determine what aquatic life is living in that environment. It’s an indicator of what kind of life is thriving there. What is Dissolved Oxygen in Water?
Dissolved oxygen in H2O (DO) is the amount of oxygen available to all living aquatic organisms necessary to survive, living in natural waters, usually measured in parts per million (ppm) used as an indicator of the health of lakes/streams.
5 ppm & higher-healthy
Below 3 ppm-concern
Below 1 ppm-hypoxic
Although Water (H2O) molecules contain an oxygen atom, this oxygen is not what is needed by aquatic organisms living in natural waters. A small amount of about ten molecules of oxygen per million of water, is actually dissolved & available in a stream or lake & used as a measure of its water quality.
What is Dissolved Oxygen
All living things need oxygen to survive. Human beings must use the oxygen that’s available within the air we breathe. In our atmosphere, oxygen makes up around 20.9 mg/l or ppm in our atmosphere, but in water;
Dissolved oxygen (DO) measures how much oxygen is dissolved in water & the amount of oxygen available to living aquatic organisms. The amount of dissolved oxygen in a stream or lake can tell much about its water quality.
It works like this water where fish and other living organisms use available oxygen that is dissolved in solution. The oxygen that’s available for them to breathe is needed for growth and health.
If there is too many fish in the water, then the fish will struggle to get what they need for survival. If there is an abundance of oxygen available in water, then more fish and other aquatic life can live there.
Most animals require DO concentrations of at least 5 mg/l or parts per million (ppm). Depending on the body of water and how much Dissolved Oxygen is available can determine what kind of aquatic life is living in that environment. Below Dissolve Oxygen levels of 3 mg/l signal distress of streams or lakes.
Dissolved Oxygen is a measure of how much oxygen is dissolved in water. This is the amount of Oxygen available for living aquatic organisms. The amount of Oxygen in a stream or a lake can tell you how healthy that stream or lake is. Dissolved oxygen refers to the level of free, non-compound oxygen present in water or other liquids.
Dissolved oxygen is oxygen molecules that have actually dissolved in water. It is not the bubbles that you may see in water, but in reality, the dissolved form of oxygen cannot be seen. Oxygen is not very soluble in water. Under normal circumstances, about 12 parts of oxygen can dissolve in a million parts of water (12 mg/liter).
Dissolved Oxygen is not bonded to any other element. Dissolved oxygen in water is the presence of these free O2 molecules within H2O. The bonded oxygen molecule in water (H2O) is in a compound and does not count toward dissolved oxygen levels. Free Oxygen that is dissolved in water is like sugar or salt that is dissolved in water when stirred and mixed.
How Does Dissolved Oxygen Get Into Water
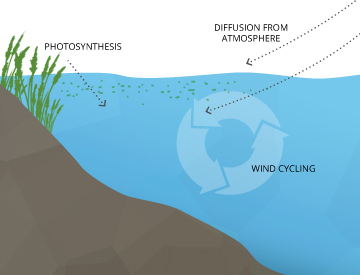 Dissolved oxygen (DO) enters the water
Dissolved oxygen (DO) enters the water
- Through contact with the air-slowly diffusing across the water’s surface from the surrounding atmosphere above
- Being mixed quickly creates aeration & water movement– Man-made waterfalls, rapids, and other aeration pumps can add Dissolved Oxygen.
- Groundwater will also contribute to Dissolved Oxygen-discharging into a stream or another body of water.
- Photosynthesis– During the process of photosynthesis, oxygen is produced as a waste product of plant & algae.
Contact from the atmospheric transfer of dissolved oxygen is the biggest way of infusing O2 into an aquatic system; the surface area to volume ratio is very important for establishing the baseline oxygen status for a given water body.
Water Movement causing aeration of water can be caused by wind creating waves, rapids, waterfalls, groundwater discharge, or other forms of running water.
Photosynthesis occurs during daylight hours and at the surface by shallow water plants and algae; a large portion of the process takes place underwater by seaweed, sub-surface algae, and phytoplankton. Light can penetrate a depth of water, though that depth varies due to dissolved solids and other light-scattering elements present in the water.
Oxygen is used up when excess organic materials, such as large algal blooms, are decomposed by microorganisms. During this decomposition process, DO in the water is consumed. Low oxygen levels often occur at the bottom of the water column and affect organisms that live in the sediments. In some water bodies, DO levels fluctuate periodically, seasonally, and even as part of the natural daily ecology of the aquatic resource.
What Affects Dissolved Oxygen Levels in Water
The amount of oxygen that can be dissolved in water or DO depends on a few Natural Factors that, include:
- Water temperature
- The number of dissolved salts present in the water (salinity)
- Atmospheric pressure
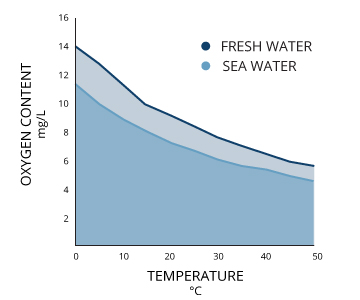 Dissolved oxygen will be higher in colder waters than in warmer ones. Less in higher water temperatures. In winter and early spring, when the water temperature is low, the dissolved oxygen concentration is high. In summer and fall, when the water temperature is high, the dissolved oxygen concentration is often lower.
Dissolved oxygen will be higher in colder waters than in warmer ones. Less in higher water temperatures. In winter and early spring, when the water temperature is low, the dissolved oxygen concentration is high. In summer and fall, when the water temperature is high, the dissolved oxygen concentration is often lower.
Dissolved oxygen will increase as pressure increases. This is true of both atmospheric and hydrostatic pressures. Water at lower altitudes can hold more dissolved oxygen than water at higher altitudes.
Dissolved oxygen decreases dramatically as salt levels increase. That is why, at the same pressure and temperature, saltwater holds about 20% less dissolved oxygen than freshwater.
Microorganisms require oxygen to decompose waste in the water. Organic waste is anything that was once part of a plant or animal, such as leaves and manure. If there is a lot of organic waste in the stream, then the microorganisms multiply and use more oxygen than can be replaced in the stream.
Phosphorous and Nitrogen are nutrients that are by-products of organic decomposition that also require oxygen to break down. In summary, colder, deeper freshwaters have the capability to hold higher concentrations of dissolved oxygen.
Human activities can add to this Organic Waste and affect the amount of dissolved oxygen in the water, including:
Organic wastes from;
• Untreated sewage
• Runoff from dairies, feedlots, farms, and other agricultural operations;
• Lawn clippings, topsoil, and other materials from around our homes;
• Land clearing man-made activities such as logging or construction;
• Stormwater runoff from agricultural fields and urban areas
Water availability is the quantity of water that can be used for human purposes without significant harm to ecosystems or other users. Consideration is given to demands from human and ecosystem needs, equitable apportionment of water among uses, and indicators of stress to the water resource ……………………………………………………………………….. Read more
Eutrophication
Eutrophication is a natural process that results from the accumulation of nutrients, particularly phosphorus, and nitrogen, in lakes or other bodies of water. Algae that feed on nutrients grow into unsightly scum on the water surface, decreasing recreational value and quality.
The excessive richness of nutrients in a lake or other body of water is frequently due to insufficient wastewater treatment or runoff from the land, which causes a dense growth of plant life, algae blooms, and death of animal life from lack of Dissolved Oxygen below 1.0 mg/l. Here is a great video that explains it.
Dead Zones are caused by an increase of nutrients mostly nitrogen & phosphorous in agricultural fertilizers & sewage runoff with natural factors playing a role in the development of excessive algae blooms that block out the sun & deplete underwater oxygen levels (eutrophication) killing aquatic life ………………………………………………………………………….. Read more
What are Water Quality Indicators?
By measuring Biological Indicators using your senses of smell and sight you will be able to get some clues and symptoms of the health of a body of water along with the ecosystem that is supported by it without testing the water. Fish & insect activity on the edge, birdlife, surface color, or sheen …………………………………………………………………………. Read more
JimGalloway Author/Editor

References: USGS Dissolved Oxygen and Water
Utah University–What is Dissolved Oxygen
