When people ask me for advice on their swimming pools or spas on where they should start at the beginning of the season, I tell them that the most important critical parameter test to run for water treatment is the total alkalinity test because low or high alkalinity can make or break your pool water chemistry. How do you Lower Alkalinity Without Lowering pH?
Running pool features, bubblers, sprayers, jets, & waterfalls, create turbulence that will remove CO2 faster raising the pool’s pH & when total alkalinity needs lowering without affecting the pH level, an acid is added, & the pool water is aerated to raise the pH level without affecting alkalinity.
The longer you operate a swimming pool the more you can how water chemistry works and the more you learn about water chemistry the more money and time you can save and that’s a good thing.
How to Lower Alkalinity Without Lowering pH
Total Alkalinity (TA)
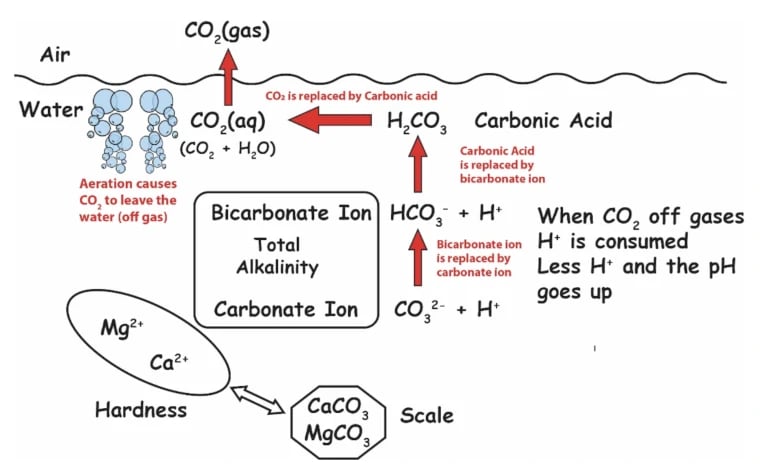
is the sum of dissolved alkali in the water; specifically, substances that can take Hydrogen ions (H+). The ability to take Hydrogen slows the drop in pH, hence alkalinity is a measure of the pH buffering capacity of water that resists a drop in pH. The opposite of alkalinity would be acidity, which resists the rise in pH
Total alkalinity and pH are so closely related that lowering alkalinity without lowering pH is pretty challenging, but not impossible. Adding an acid to water will lower the total alkalinity, but it will also lower the pH levels somewhat.
If the total alkalinity needs lowering without affecting the pH level, an acid is added, and straight after the water is aerated to raise the pH level without affecting the alkalinity. However, in reality, you cannot lower or raise one without it affecting the other.
When reducing alkalinity with acid most time pH will also be reduced. But there is a way to minimize this: create turbulence in the pool so CO2 leaves as fast as possible. This means accelerating the loss of CO2 so the pH rises back up and leaves the pool water quickly.
This means running water features, bubblers, sprayers, jets, waterfalls, and anything else you can. We have seen people get creative with air compressors and diffusers to create bubbles. The point is, you want the CO2 out faster.
What Causes Low Alkalinity in Pools?
- Rain can be acidic
- Increase in swimmer load
- Topping your pool off with H2O that has low alkalinity after backwashing/wasting
- Chlorine-Chlorine is acidic
- High H2O evaporation-losing H2O can dilute TA
- Pool H2O agitation-H2O agitation increases the loss of CO2 affecting TA
- Lotions & detergents-Keep a pool shower ……………………………………………………………………………………. Read more
Controlling pH in a Swimming Pool
Technically the pH is the concentration of Hydrogen ions, expressed logarithmically between 0 and 14. But that’s complex and hard to wrap our heads around. So for practical purposes, a better way to understand pH is by thinking about the amount of carbon dioxide (CO2) dissolved in your water.
The more dissolved CO2 in your water, the lower the pH, and vice versa. This should explain why CO2 injectors lower pH, and why aeration (such as splash features, spillovers, and spa jets) raises pH.
If you’ve got water, any gas dissolved in that water has to be the exact proportionate pressure of that same gas above the water. And it’s not necessarily equal, it’s proportionate. In this case, the gas we care about is carbon dioxide. Why CO2?
Because the amount of CO2 is going to help you determine the pH. The less CO2 in your water, the higher your pH. The more CO2 dissolved in your water, the lower your pH, because you’ll have more carbonic acid.
If you have waterfall spray features, splash features, vanishing edge, aeration of any kind, bubbles, whatever. Your pH rises in those pools pretty quickly. The reason for that is those bubbles accelerate the loss of CO2. Your CO2 leaves, and your pH goes up.
If you want to reduce pH, you can inject CO2. The pH goes down, but the alkalinity doesn’t. And the reason the alkalinity doesn’t is because you didn’t do anything to the alkalinity.
If you need more CO2 to lower your pH, how does HCl find CO2? Well, it takes it from alkalinity. The way I describe it it’s not chemically accurate, but it’s a good visual. Does it burn through alkalinity? The acid will burn through alkalinity, convert it to carbonic acid, and boom. Your pH goes down.
The amount of carbon dioxide in water determines the pH of the water. The more CO2, the lower the pH, and vice versa. A pool’s pH will naturally rise over time, thanks to CO2 leaving the water.
Henry’s Law of solubility of gases explains why CO2 leaves until it is in equilibrium with the atmosphere. When that equilibrium is reached, the water is at what we call its “pH ceiling”. This ceiling is determined by the carbonate alkalinity in the pool water.
Controlling pH is crucial in various applications, including water treatment, aquaculture, hydroponics, and swimming pool maintenance. Here’s a list of methods commonly used to control pH levels:
- Chemical Adjusters:
Acids: Adding acids like hydrochloric acid (muriatic acid), sulfuric acid, or phosphoric acid can lower pH levels by neutralizing alkalinity.
Bases: Adding bases like sodium hydroxide (caustic soda) or sodium carbonate (soda ash) can raise pH levels by neutralizing acidity.
- Buffering Agents:
Bicarbonates and Carbonates: These compounds act as pH buffers, resisting changes in pH by absorbing excess acids or bases. Common examples include sodium bicarbonate (baking soda) and sodium carbonate (soda ash).
Borate Compounds: Borates help stabilize pH and prevent fluctuations, particularly in swimming pools.
- CO2 Injection:
Injecting carbon dioxide (CO2) into water lowers pH by forming carbonic acid (H2CO3) through the reaction between CO2 and water. This method is commonly used in aquariums and hydroponic systems.
- Aeration:
Aeration involves exposing water to air, allowing carbon dioxide (CO2) to escape, which can raise pH levels by reducing carbonic acid concentration. It’s often used to increase pH in ponds and aquaculture systems.
- Ion Exchange Resins:
Ion exchange resins can selectively remove ions responsible for pH fluctuations, helping to stabilize pH levels in water treatment systems.
- Reverse Osmosis (RO):
RO systems can remove dissolved minerals and ions, including those affecting pH, resulting in water with more stable pH levels.
- Natural Substances:
Peat Moss: Adding peat moss to water can lower pH levels naturally due to its acidic properties, making it useful in aquariums and freshwater systems.
Driftwood: Similar to peat moss, driftwood releases tannins that can lower pH levels in aquariums.
- Biological Processes:
Beneficial bacteria in biological filtration systems can influence pH by consuming organic matter and producing acidic or basic byproducts. This method is common in aquaponics and natural pond systems.
- pH-Buffering Substrates:
Substrates like crushed coral or limestone can act as pH buffers, gradually releasing alkaline compounds to maintain stable pH levels in aquariums or ponds.
- Monitoring and Adjustment:
Regular monitoring of pH levels using pH meters or test kits is essential for maintaining optimal conditions. Adjustments can be made as needed based on testing results.
When choosing a method to control pH, consider factors such as the specific requirements of the system, the desired pH range, and the potential impact on aquatic life or plant growth. It’s also important to follow proper safety protocols when handling chemicals and to regularly monitor pH levels to ensure water quality remains within acceptable limits.
How do you adjust High pH with High Alkalinity?
- Run pool pump-bypass filter
- Test Total Alkalinity
- Calculate Muriatic Acid addition with a Pool Alkalinity Calculator
- Mix Muriatic Acid in a bucket of pool H2O
- Evenly distribute by walking around the perimeter of the pool
- Wait for 6-8 hrs
- If TA is 80-130 ppm.……………………………………………………………………………………Read more
Henry’s Law describes the solubility of a gas in a liquid at a constant temperature. It states that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid. In other words, as the pressure of the gas above the liquid increases, the amount of gas that can dissolve in the liquid also increases.
Mathematically, Henry’s Law is often expressed as:
�=�⋅�
Where:
- � is the concentration of the dissolved gas in the liquid (typically in mol/L or mg/L),
- � is Henry’s law constant, which is specific to the gas and the solvent at a given temperature,
- � is the partial pressure of the gas above the liquid.
Henry’s Law is essential in various fields, including chemistry, environmental science, and engineering. It helps explain phenomena such as the gas exchange between the atmosphere and bodies of water, the dissolution of gases in beverages, and the behavior of gases in industrial processes like gas absorption and stripping operations.
Understanding Henry’s Law is crucial for predicting the behavior of gases in different systems and optimizing processes where gas-liquid interactions play a significant role. Additionally, deviations from ideal behavior, such as temperature and pressure effects, can be accounted for by modifying Henry’s Law with additional constants or equations, providing a more accurate representation of real-world conditions.
Conclusion:
In conclusion, lowering alkalinity without affecting pH is essential for maintaining water quality in various environments. By utilizing methods such as aeration, acid buffers, and natural processes, it’s possible to achieve this balance effectively. Regular monitoring of water chemistry and adjustments based on testing results are crucial for ensuring optimal conditions for aquatic life and other applications. Additionally, consulting with experts or professionals can provide valuable guidance in selecting the most suitable approach for specific needs. Overall, implementing these strategies can lead to a healthy and stable aquatic environment while avoiding potential complications associated with pH fluctuations.
Does Rain Lower Pool Alkalinity?
Yes taking a defensive approach, a pool owner can ward off the effects of added acidity, phosphorus & organics affecting the chemistry in a well-balanced pool before it rains by building the pool H2O up:
- Alkalinity levels up to 20-30 ppm
- Raising pH to the 7.6-7.8 range
- Raise Chlorine Levels
- Run Pool Filter ………………………………………………………………………….. Read more

References:
AQUA Magazine- Acid Myths and Taboos You Should Break
FAQ’s