- Ocean water is salty due to dissolved minerals
- Rain erodes rocks, releasing salts into rivers
- Rivers carry salts to oceans
- Evaporation leaves salts behind, increasing salinity
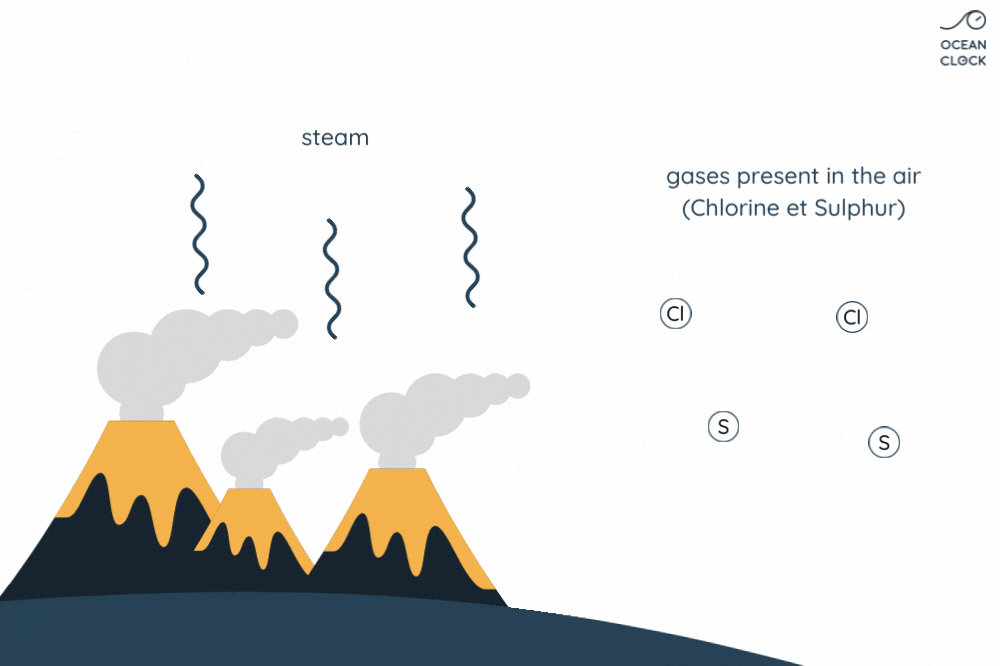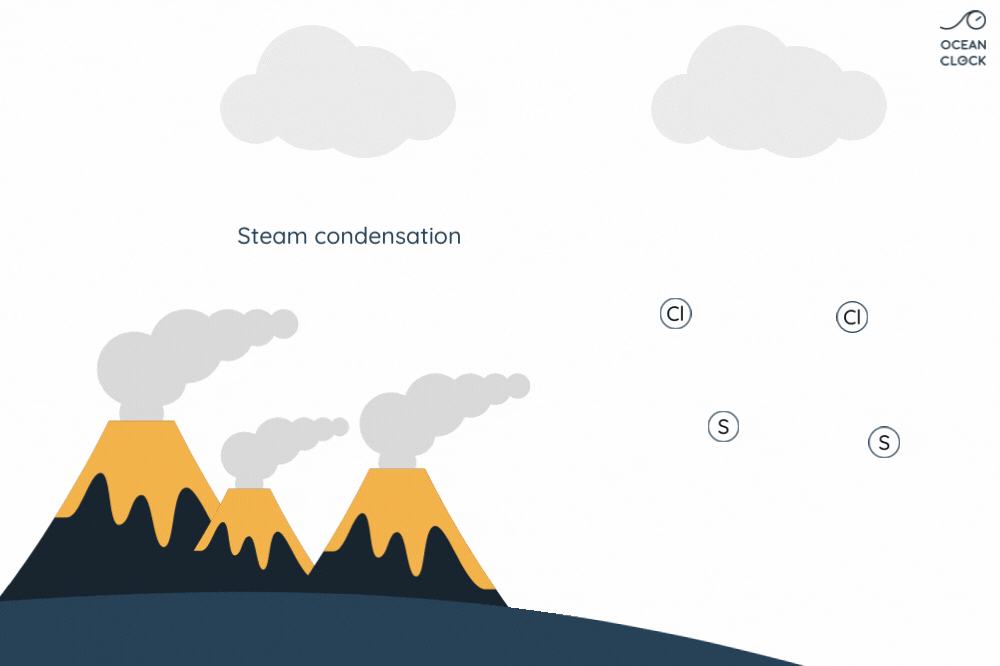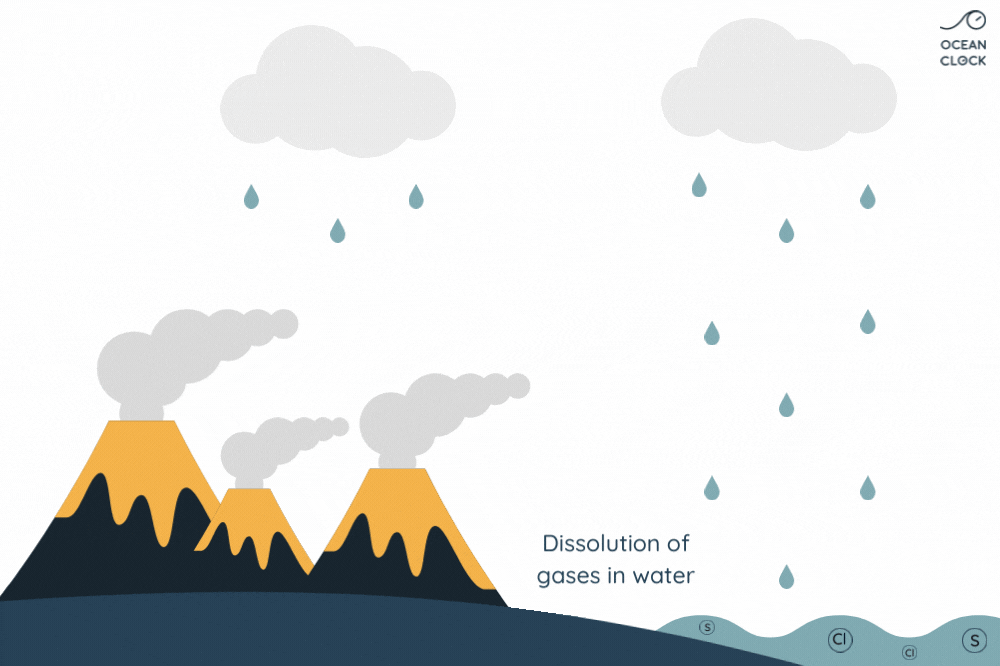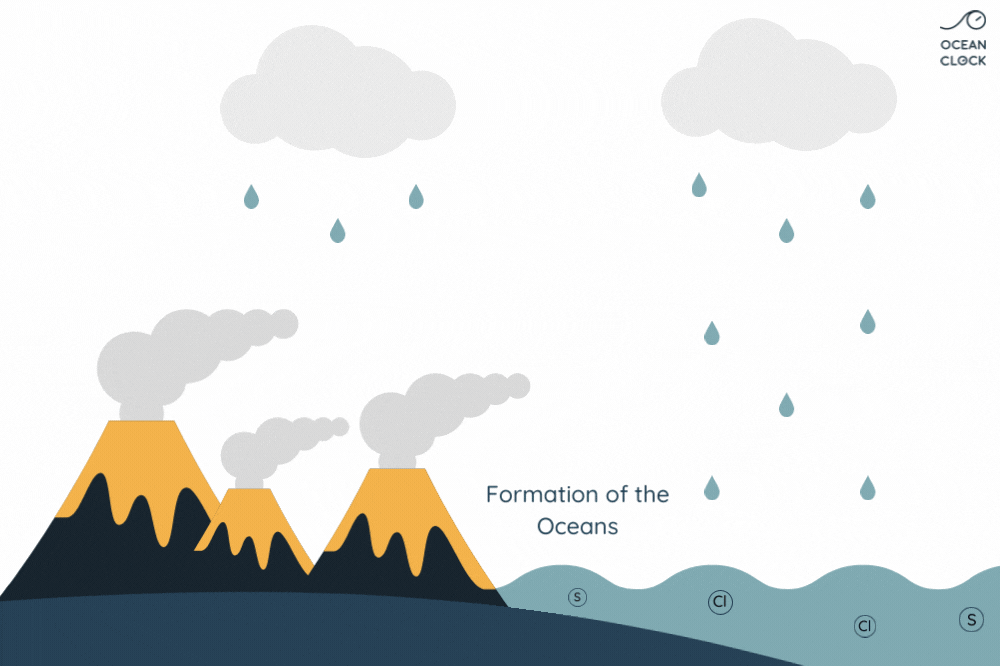
- Underwater volcanic activity adds minerals
- Hydrothermal vents release salts
- Salinity accumulates over time
- Freshwater inflows dilute salinity
When freshwater flows into the ocean, it carries salts and minerals with it. More salts and minerals are added from seafloor vents. Deep in the ocean, water seeps into cracks in the Earth’s crust. There, it’s heated by magma. Hot water dissolves salts and minerals from the rock.
Why is Ocean Water Salty?
The Science Behind Ocean Salinity
Ever wondered why ocean water is salty? It’s mainly caused by rain washing mineral ions from the land into water bodies. These mineral ions, or salt, in the ocean, come from rocks on land. The levels of salt in the ocean have built up over time, making it increasingly saline.
The process starts when rain falls on rocks, breaking them down and releasing ions. These ions are carried by rivers and streams into the ocean. Over countless years, the continuous addition of minerals has made the oceans salty. The science behind it also reveals that saline water in the sea is a complex mixture, influenced by various factors, including the role of hydrothermal vents.
These underwater vents release minerals into the ocean, affecting its salinity levels. Using data and science, we can see how salt content in sea water varies across different ocean parts. The study of salinity is crucial for understanding oceanography and the various factors that affect salty oceans. Curious about more details? Learn about it through USGS studies.
How Water from Rivers Entering the Ocean Contributes
Many people wonder why ocean water is salty. One significant reason is the way water from rivers contributes to the salinity of sea water. When rivers flow over the earth’s surface, they collect minerals and salts from the soil and rocks they pass over. As this water eventually makes its way to the ocean, it carries these salts along.
Although river water itself isn’t very salty, the continuous flow of water into the ocean leads to a gradual build-up of ocean salt. This process helps maintain the saline nature of seawater. Coastal areas are especially impacted by this phenomenon.
Additionally, marine life and activities happening in these coastal regions contribute further to keeping ocean water salty. Understanding this relationship gives insights into why even freshwater rivers play a role in maintaining the saline balance of our oceans. Even though the water coming from rivers isn’t salty, it’s their journey through the earth and eventual destination into the ocean that results in the salty water we find in seas and oceans.

The Role of Hydrothermal Vents in Ocean Salt Levels
The salt that dissolves in ocean water from these vents contributes to the overall salinity of seawater. Interestingly, these vents provide not just salt but also other minerals essential for marine life. So, next time you wonder why ocean water is salty, remember the hidden hydrothermal vents working deep below. This knowledge can help us understand more about Earth’s oceans and the factors that make seawater saline. Want access to more about ocean salt and salinity? Keep exploring to uncover more fascinating Earth science!
Understanding Sea Water and Its Salt Content
Why is ocean water salty? The salinity of sea water is an interesting topic in science that many students find fascinating. Oceans are salty because minerals and salts are constantly dissolving into the water from various sources. River water, for instance, flows into the oceans and brings along small amounts of minerals, which contribute to the ocean water’s salinity. Additionally, hydrothermal vents on the ocean floor release minerals and salts, making the water salty.
Waves and currents help mix the salts throughout the oceans, maintaining the salinity levels. The connection or link between these factors creates a balanced salty environment in our seas. By understanding the science behind ocean salinity, we can better appreciate the complexity of our planet’s bodies of water. The process of how water becomes salty is a great example of the intricate interactions within our natural world.
Factors Affecting Salty Oceans
Many factors contribute to the salty nature of our oceans. One major factor is the dissolution of minerals from rocks, particularly those containing salt. When rivers flow into the ocean, they carry small amounts of dissolved salts, which accumulate over time, making the ocean saltier. Evaporation also plays a crucial role; as ocean water evaporates, the salinity increases because the salt is left behind.
Hydrothermal vents on the ocean floor further add to the salt content by releasing minerals directly into the water. Wind and wave actions mix the saline water, distributing salt more evenly across the ocean. Interestingly, the USGS studies show that the salinity levels can vary in different parts of the ocean due to temperature and location.
In colder regions, ocean water tends to be less salty compared to warmer regions. Resources like Britannica provide detailed information about these complex interactions. Understanding these factors helps us appreciate why the sea is salty, emphasizing the expansive nature of our planet’s oceans.
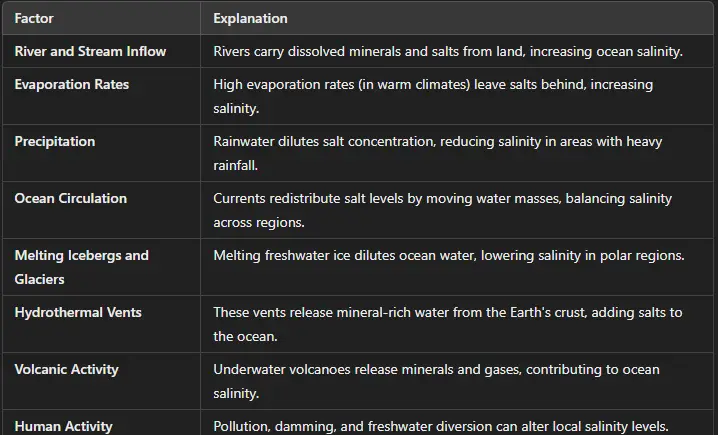
Here’s a table outlining the factors affecting ocean salinity:
Which Ocean is Saltiest?
The Atlantic Ocean is the saltiest. Its higher salinity is primarily due to high evaporation rates, limited freshwater inflow from rivers, and lower precipitation compared to other oceans. The warm waters of the subtropical regions in the Atlantic experience intense evaporation, leaving behind more concentrated salts. Additionally, the Mediterranean Sea, which connects to the Atlantic, also has high salinity, further contributing to the overall salt levels in the Atlantic Ocean.
How is Salinity Measured?
Interested in Oceanography: Learn More About USGS Studies
If you’re curious about why the ocean is salty, you’re in for a treat! Oceanography is the study of our planet’s vast and mysterious oceans. With 71% of the Earth’s surface covered by oceans, it’s crucial to understand their composition and behavior.
The USGS (United States Geological Survey) is at the forefront of studying the ocean and its various characteristics, including why the ocean water is so salty. If you’re fascinated by the ocean, diving into USGS studies can be incredibly enlightening.
They conduct extensive research on how the inflow of freshwater from rivers impacts ocean salinity and the role hydrothermal vents play in adding minerals to the ocean. These studies help us grasp the dynamic nature of ocean water and its saline content.
Want to know more? Exploring USGS research will give you deeper insights into factors affecting salty oceans, such as evaporation and rainfall. So, if the ocean’s mysteries captivate you, the USGS has a wealth of information to satisfy your curiosity about the ocean.
Conclusion:
Ocean salinity is affected by several natural processes, including the weathering of rocks, river inflow, evaporation, and volcanic activity on the ocean floor. While precipitation, melting ice, and ocean currents can cause variations in salinity in different areas, the Atlantic Ocean is the saltiest due to its high evaporation rates and limited freshwater input. Salinity is crucial for ocean circulation and marine life, and it is typically measured using methods like electrical conductivity and refractometry. Understanding salinity is key to studying the Earth’s climate, ecosystems, and the water cycle.
References:
USGS- Lifespan of Salt Marsh Units
FAQ’s
- Why is ocean water salty but freshwater lakes are not?
- Ocean water is salty because it accumulates dissolved minerals, particularly salt, from rivers, streams, and undersea processes. In contrast, lakes often have outflows, so salts do not build up as much.
- What is the average salinity of ocean water?
- The average salinity of ocean water is about 35 parts per thousand (ppt), meaning there are 35 grams of salt for every kilogram of seawater.
- Which ocean has the highest salinity?
- The Atlantic Ocean has the highest salinity among the world’s oceans, particularly in its subtropical regions where evaporation is high.
- How does salinity affect marine life?
- Salinity influences the types of organisms that can live in different parts of the ocean. Some species are adapted to high salinity, while others thrive in lower-salinity environments, making it a key factor in marine ecosystems.
- Can humans drink ocean water by removing the salt?
- Yes, through a process called desalination, salt can be removed from seawater, making it safe for drinking, though this process can be energy-intensive and expensive.
- Does climate change impact ocean salinity?
- Yes, climate change can alter salinity patterns through changes in evaporation rates, precipitation, and melting ice, which affects the distribution of freshwater in the oceans.
- How do scientists measure salinity in the ocean?
- Scientists measure salinity using tools like conductivity meters, refractometers, and chemical titration, often reported in practical salinity units (PSU) or parts per thousand (ppt).