Dissolved oxygen is a crucial aspect of aquatic systems from the smallest streams, creeks, lakes rivers, and oceans providing a necessary element for the survival of aquatic life. How Does Dissolved Oxygen Get Into Water?
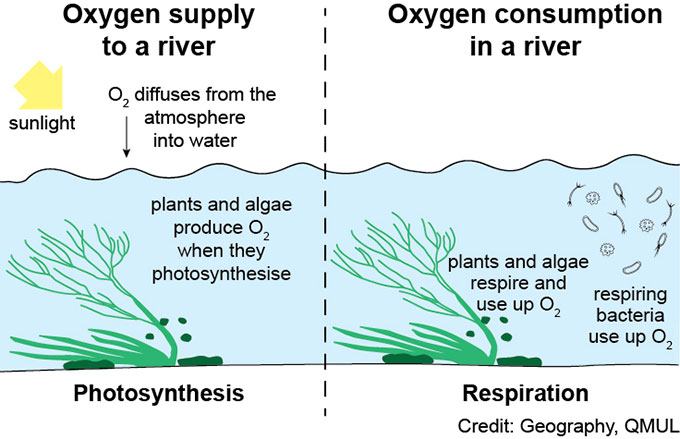
Oxygen enters into water primarily through two processes :
Photosynthesis- Dissolved oxygen enters H2O through the air or as a plant byproduct.
Diffusion-From the air, oxygen can slowly diffuse across the water’s surface from the atmosphere, or be mixed in quickly through aeration (natural or man-made)
The amount of dissolved oxygen in water is influenced by several factors. Three key factors that impact dissolved oxygen levels are water temperature, salinity, and atmospheric pressure.
Introduction:
When we talk about oxygen in water, our minds often acknowledge the life it sustains, such as fish and other marine organisms. But have you ever wondered how this vital element ends up dissolved in water? The presence of dissolved oxygen in water is much more than a mere cocktail of H2O and O2; it’s a process linking the aquatic environment with the atmosphere. This article will help explain how oxygen is taken from the air to a dissolved state in water, helping you comprehend this essential and complex ecosystem process.
How Does Dissolved Oxygen Get Into Water
Understanding how dissolved oxygen gets into water is fundamental for grasping aquatic life’s survival. Oxygen enters into water primarily through two processes –
- photosynthesis
- diffusion from the atmosphere.
Oxygen gets into water when atmospheric oxygen is directly absorbed. During daylight hours, underwater photosynthesis by plants contributes to the levels of dissolved oxygen in water bodies. As part of the process, plants obtain oxygen and release it into the water.
Dissolved oxygen is critical for organisms living underwater. Be it a vast sea or a small creek; water-dwelling life forms depend on the availability of dissolved oxygen. How does dissolved oxygen help them? It’s simple. Similar to how humans inhale oxygen from the air for survival, aquatic species rely on the oxygen dissolved in water to breathe.
Dissolved oxygen enters water through various processes. It can slowly diffuse across the water’s surface from the surrounding atmosphere. Additionally, oxygen can be introduced into the water through natural or man-made aeration. Aeration methods include techniques like oxygen saturation technology, submersed aerators, floating fountains, and nanobubble technology.
In aquatic environments, oxygen can also be a byproduct of photosynthesis from aquatic plants, which release oxygen as they undergo photosynthesis.
The oxygen that is dissolved in water is readily used by aquatic organisms. This shows that oxygen is not merely a passive molecule floating around. Instead, it’s an active component of an aquatic ecosystem. The movement of waves and currents helps the process where oxygen gets into water. As water moves, it comes into contact with air, enabling the diffusion of oxygen into water.
The amount of dissolved oxygen varies, and it’s dependent on factors such as
- water temperature,
- salinity
- pressure
More oxygen is dissolved in cold water than in warm water. Hence, the dissolution of oxygen into water is impacted by the water’s physical characteristics. The concentration of oxygen also affects the species variety. For instance, trout and stoneflies require high levels of dissolved oxygen, whereas worms and black fly larvae can survive even on lower levels.
Understanding how dissolved oxygen enters and is utilized in water is essential for maintaining healthy aquatic ecosystems. The diffusion from the atmosphere and the process of photosynthesis are key for integrating oxygen into water bodies. Dissolved oxygen is crucial for underwater life forms, making it a key topic of study for environmental scientists.
Dissolved Oxygen: Oxygen Demand and Levels in Water
When it comes to oxygen levels in water, a variety of factors determine the concentration. From aspects such as water temperatures to the biological oxygen demand present, oxygen concentration significantly impacts overall water health. Accordingly, it’s essential to comprehend the oxygen demand, the levels of oxygen, and how these levels can be low or high.
Dissolved oxygen is a crucial aspect of aquatic systems, providing a necessary element for the survival of aquatic life. However, the oxygen levels in water aren’t consistently abundant. Even slight changes in oxygen concentration can influence the entire ecosystem.
Let’s look a little deeper into understanding these variations and how dissolved oxygen makes its way into water. The knowledge will help us figure out ways to maintain appropriate water levels and ensure environmental sustainability.
Given the importance of oxygen levels, the demand for oxygen or the oxygen demand plays a significant role in dictating the water’s health status. Oxygen demand indicates the amount of oxygen that the water habitat necessitates for its organisms to survive. When this oxygen demand is high, the oxygen levels can be alarmingly low.
However, it’s essential to note that low oxygen levels don’t necessarily mean a habitat is unhealthy. In some waters, a low level of about 1 to 2 mg of oxygen per liter(mgl) can be perfectly normal. Meanwhile, in others, a level of only 5 to 6 mgl could denote a dangerously low oxygen concentration.
The amount of dissolved oxygen in water is influenced by several factors. Three key factors that impact dissolved oxygen levels are water temperature, salinity, and atmospheric pressure.
- Water Temperature: The temperature of the water plays a crucial role in determining dissolved oxygen levels. Warmer water has a lower capacity to hold oxygen, so as water temperature increases, the amount of dissolved oxygen decreases. Conversely, cooler water can hold more dissolved oxygen. This temperature-dependent solubility of oxygen affects aquatic ecosystems, as many organisms rely on dissolved oxygen for respiration.
- Salinity: Salinity refers to the concentration of dissolved salts in water. Freshwater can hold more oxygen than saltwater, so higher salinity levels can lead to lower dissolved oxygen concentrations. This is why aquatic environments in saline waters may have lower oxygen levels compared to those in freshwater ecosystems.
- Atmospheric Pressure: Atmospheric pressure can influence the solubility of oxygen in water. Higher pressure levels, such as those found at greater depths in bodies of water, can increase the amount of dissolved oxygen. Conversely, lower atmospheric pressure, which can occur at higher altitudes, may result in decreased oxygen solubility.
These factors interact and fluctuate in natural aquatic systems, impacting the availability of dissolved oxygen and, in turn, affecting the survival and distribution of aquatic life.
Depletion of Oxygen: The Factors and the Process
Water bodies, such as lakes and rivers, play a crucial role in sustaining life on Earth. They’re teeming with diverse species, many of which depend on a key factor for survival – oxygen. However, there are instances when the level of oxygen diminishes, leading to low oxygen conditions in these water bodies. This phenomenon, known as the depletion of oxygen, is primarily triggered by certain conditions and processes that impact surface waters, both freshwater and marine.
The temperature of the water is one noteworthy factor that influences oxygen depletion. It’s worth noting that the water’s temperature can either increase or decrease the amount of oxygen that can be dissolved. As the temperature increases, the oxygen-holding capacity of water reduces.
So, higher temperatures mean lower oxygen levels. This is further complicated as surface waters, which are exposed to sunlight and atmospheric heat, tend to have higher temperatures. In fact, surface waters can potentially experience drastic fluctuations in temperature, which could lead to a sudden drop in the oxygen level, thereby disrupting the water quality and the ecosystem living within.
Simultaneously, processes in the water also play a part in reducing the available amount of oxygen. Biological activities, like decomposition and photosynthesis, can severely impact the water’s oxygen level. In areas where there is excess organic material, decomposition occurs at a higher rate, which in return consumes more oxygen. This can lead to further reduction in the amount of oxygen in surface waters and increase the rate of depletion of oxygen. This process is a real threat to aquatic life, posing risks for survival and growth.
Identifying how oxygen gets into the water in the first place gives us a fundamental understanding of how it can also get depleted. It is imperative to monitor these factors and processes to maintain water quality and ecological balance in our water bodies. Through proactive research and effective management, we can ensure that oxygen depletion in our waters is minimally detrimental to aquatic life.
How Do Areas Become Oxygen-Depleted and its Impact on Water Quality
When we’re exploring oxygen-depleted areas, it’s important to consider the factors that lead to this issue. Key amongst these is temperature. When water temperature skyrockets, there’s a direct impact on oxygen levels and consequently, the quality of water.
Temperature is a pivotal factor: as it increases and fluctuates, the ability of water to hold oxygen decreases, leading to lessened water quality. As such, areas witnessing unregulated temperature spikes often suffer from depleted levels of oxygen.
This oxygen-depletion cycle, in turn, results in less-than-optimal conditions for aquatic life, especially fish. Fish in temperature-stressed waters struggle to get sufficient oxygen.
When oxygen levels dip, the overall water quality plunges too. At this point, let’s highlight how temperature, oxygen, and fish are intimately connected. Areas that manage their water temperature are likely to contain a thriving fish population – proof of excellent water quality.
However, the issue doesn’t just stop at temperature or fish. Water quality is an overall evaluation of the health of water in a given area. Lessons from recorded data thus far only serve to emphasize the role temperature plays in ensuring oxygen-rich water bodies.
So, what can be done to counteract oxygen depletion? Based on our understanding, it’s crucial to monitor water quality, temperature, and oxygen levels regularly. In doing so, we can ensure that fish populations remain healthy and our areas of interest stay oxygen-abundant.
Furthermore, we’ve also learned that areas responding proactively to temperature spikes can prevent significant harm to their water bodies. Such is the impact of temperature on both the oxygen and water quality of an area. To sum up, let’s emphasize doing our part to preserve the quality of water in our areas.
Causes of Low Dissolved Oxygen in Water:
Low dissolved oxygen (DO) in water can be attributed to several factors, including:
- High Temperatures: Warm water has lower oxygen solubility than cold water. When water temperatures rise, it can lead to reduced dissolved oxygen levels, impacting aquatic life.
- Excessive Nutrients: High nutrient levels, particularly phosphorus and nitrogen, can spur the growth of algae and aquatic plants. As these organisms decompose, they consume oxygen, causing a decline in dissolved oxygen levels.
- Decaying Organic Matter: Organic matter from dead algae, plants, and other organic materials in the water can decompose, consuming dissolved oxygen in the process and leading to oxygen depletion.
- Bacterial Decomposition: Bacteria play a crucial role in breaking down organic matter, but this decomposition process requires oxygen. When there’s an excess of organic material, bacterial decomposition can deplete available oxygen.
- Turbidity and Pollution: Increased turbidity or the presence of pollutants in water can limit the penetration of sunlight, affecting the photosynthetic activity of aquatic plants and algae. This, in turn, affects oxygen production and can reduce dissolved oxygen levels.
Measurement of Dissolved Oxygen in Water: How & Why
Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in the water – the amount of oxygen available to living aquatic organisms. The amount of dissolved oxygen in a stream or lake can tell us a lot about its water quality.
Rapidly moving water, such as in a mountain stream or large river, tends to contain a lot of dissolved oxygen, whereas stagnant water contains less. Bacteria in water can consume oxygen as organic matter decays. Thus, excess organic material in lakes and rivers can cause eutrophic conditions, which is an oxygen-deficient situation that can cause a water body to “die.”
Dissolved oxygen in surface water is used by all forms of aquatic life; therefore, this constituent typically is measured to assess the “health” of lakes and streams.
Although water molecules contain an oxygen atom, this oxygen is not what is needed by aquatic organisms living in natural waters. A small amount of oxygen, up to about ten molecules of oxygen per million of water, is actually dissolved in water.
Oxygen enters a stream mainly from the atmosphere and, in areas where groundwater discharge into streams is a large portion of streamflow, from groundwater discharge. This dissolved oxygen is breathed by fish and zooplankton and is needed by them to survive.
Dissolved oxygen, a seemingly mysterious term, merely refers to the level of oxygen that is found dissolved in water. It’s a critical factor, not just for aquatic life, but for the health of water bodies on the whole. You may wonder, why do we measure dissolved oxygen levels? How is this monitoring even accomplished? Whether it’s the vast ocean expanse, a gentle babbling brook, or the water from your tap, dissolved oxygen plays a vital role.
The significance of dissolved oxygen in water can’t be understated. Simple as it may sound, water is life, and this dissolved oxygen is a lifeblood for many organisms living below water surfaces. Without it, they do not survive. Just like on the land, where the right amount of oxygen is critical for survival, it’s equally crucial in water. Too little or too much oxygen and aquatic habitats could become uninhabitable.
That’s where the measurement of dissolved oxygen comes into play. It gives researchers, environmentalists, and even savvy homeowners the necessary information to identify changes in water quality. Regular measurements also help determine if water is clean or whether further action needs to be taken to improve its quality.
Atlas of USGS Methods: Dissolved Oxygen Measurement Tools & Model
Dissolved oxygen’s essential role in the health of water bodies can’t be understated. It’s imperative to possess a comprehensive understanding of the structure and properties of dissolved oxygen and how it relates to the overall hydrological science. US Geological Survey, or USGS, employs state-of-the-art tools and developed models to measure this critical water component, enabling us to do more with the data we collect.
For example, as part of their Atlas of Methods, USGS provides tools to measure dissolved oxygen in various water bodies. Not only does this tool measure the amount of dissolved oxygen present, but it also helps scientists understand the factors that cause its levels to fluctuate. After all, the demand for and levels of dissolved oxygen in the waters can change significantly due to natural and man-made influences.
Dissolved Oxygen measurement tools are vital in understanding these fluctuations. Studying the changes in dissolved oxygen and understanding how it gets into the water is a significant aspect of water science. With technological advancements, the data generated from these tools has improved, leading to more accurate and information-rich results. This has broadened the scope of what we can do with this information.
Dissolved oxygen depletion is one of the major threats to the aquatic environment. It generally happens due to microbial processes, like the decomposition of organic matter in the absence of oxygen. These areas where oxygen is markedly reduced or nearly absent can have severe impacts on water quality – killing fish and aquatic life, and leading to a cascading effect throughout the ecosystem.
Having a dedicated model, like the one here on Amazon or USGS offers, helps to quickly identify the onset of such conditions. By effectively measuring dissolved oxygen, we’re better equipped to protect our waters. Ultimately, knowing how oxygen is dissolved and distributed in the water is integral to preserving and improving the state of our global aquatic habitats.
Insights into Water Scarcity: Is it Linked with Dissolved Oxygen Levels
Water scarcity is an undoubtedly serious issue that’s raising concerns around the world, yet what’s often overlooked is its intricate link to dissolved oxygen levels. You may be wondering, do these two resources share a relationship? Indeed, they do, and understanding this link requires grasping how oxygen gets into water.
Dissolved oxygen enters water predominantly through direct absorption from the atmosphere, present in both shallow and deep waters, ensuring life beneath the surface continues to thrive. This continuous observation of oxygen levels is essential to ensure water health, which is increasingly crucial amidst water scarcity challenges.
The lower the oxygen level in waters, the higher the likelihood of the environment becoming oxygen-depleted. An area with low oxygen, whether due to natural progressions or mankind’s impact, undergoes a significant change in water quality. Such depletion is often a direct response to various factors, mostly human-dependent like pollution, that disrupt the process of dissolved oxygen entering the water.
Notably, even though oxygen is absorbed from the atmosphere into water effortlessly, maintaining optimum levels in our waters can be drastically hindered by these disruptors. So, how do we ascertain that sufficient oxygen levels are maintained to combat water scarcity?
Monitoring dissolved oxygen is a task that involves a specific set of tools and models. Measuring the presence of oxygen levels in water is vital. Thanks to resources such as the Atlas of USGS Methods, we have access to effective dissolved oxygen measurement tools and model systems.
Each method is designed to provide accurate dissolved oxygen levels, which assist in implementing strategies to manage and combat water scarcity effectively. While addressing this scarcity is an uphill battle, understanding and maintaining optimal oxygen levels in water resources affords us a step in the right direction. Therefore, as we navigate through the challenges of water scarcity, keeping a close eye on dissolved oxygen levels is indeed part of the solution. Recognizing this link invites us to think creatively about how we approach water resources and opens new paths for combating scarcity.
Diving into the Bottom: Role and Presence of Dissolved Oxygen
So, how do we dive into the bottom of understanding dissolved oxygen’s role and presence? It’s easy to overlook the vital part played by dissolved oxygen in waters, but it’s actually at the core of aquatic life. With its invisible presence, dissolved oxygen supports fishes, animals, and the entire under-the-surface ecosystem. Levels of dissolved oxygen in water are an organic part of the environment in which these species exist.
Just like oxygen enters our bodies when we breathe, it’s able to enter the water in two main ways. One is through the air; oxygen naturally diffuses into the water from the atmosphere, particularly at the water’s surface. Another way oxygen enters is via photosynthesizing plants and algae, which give off oxygen as a waste product. The oxygen then becomes dissolved in the water, where it can be used by aquatic animals.
Fish, in particular, depend on sufficient levels of dissolved oxygen to survive. Too little oxygen, and they struggle to breathe; too much, and can harm their gills. The levels of dissolved oxygen also affect whether fish and other animals can successfully reproduce. The babies of many species, from fish to birds, depend on aquatic environments with high enough levels of dissolved oxygen.
Yet, the dissolved oxygen levels can also fluctuate. Variations in temperature, salinity, and pressure affect the amount of dissolved oxygen that water can hold. Groundwater naturally has less oxygen compared to surface water, due to its lack of contact with the atmosphere, thus limited capacity for gases to enter.
With these fluctuations in values, fishes and other aquatic animals have to adapt or migrate to regions where they can find the right levels of dissolved oxygen. If this isn’t possible, they may not survive. This is particularly concerning in growing instances where pollution or other human interference depletes the oxygen available in a pond or other bodies of water, causing a significant decrease in the dissolved oxygen available for aquatic life.
So, again, the presence of dissolved oxygen is a crucial piece that keeps the puzzle of life under the waters flowing. Without it, the striking beauties that thrive at the bottom wouldn’t exist.
Conclusion:
In conclusion, the process of how dissolved oxygen gets into water is both fascinating and vital. It primarily occurs through aeration and photosynthesis. Oxygen enters water by direct absorption from the atmosphere, typically when water moves, creating turbulence. During photosynthesis, aquatic plants release oxygen into the water, contributing significantly to oxygen levels. Understanding these processes gives us a deeper insight into aquatic ecosystems and their management. It’s a reminder of how interconnected our world truly is and how vital these processes are for the survival of aquatic life.

References:
USGS- Dissolved Oxygen
EPA- Dissolved Oxygen in Water
FAQ’s
Q: How does oxygen enter into the water?
A: Oxygen enters into water primarily through two processes – photosynthesis, and diffusion from the atmosphere. During daylight hours, underwater photosynthesis by plants contributes to the levels of dissolved oxygen in water bodies. As part of the process, plants obtain oxygen and release it into the water.
Q: Why is dissolved oxygen important for underwater life?
A: Dissolved oxygen is particularly crucial for underwater life forms. Aquatic species rely on the oxygen dissolved in water to breathe, similar to how humans inhale oxygen from the air for survival.
Q: Does the quantity of dissolved oxygen impact different species in different ways?
A: Yes, the quantity of dissolved oxygen can significantly affect different water species. For instance, trout and stoneflies require significantly high levels of dissolved oxygen, whereas worms and black fly larvae can survive even on lower levels.
Q: How is the concentration of oxygen in water maintained?
A: The concentration of oxygen in water is influenced by several factors such as water temperature, salinity, and pressure. As a result, the dissolution of oxygen into water is impacted by the physical characteristics of the water.
Q: What happens when there is a depletion of oxygen in water bodies?
A: Depletion of oxygen in water bodies disrupts the water quality and the ecosystem living within. It impacts the oxygen-holding capacity of water, thereby affecting the survival and growth of aquatic life. This process is particularly harmful to fish and other aquatic species as it reduces their chances of survival.

