The amount of oxygen dissolved in a stream or lake’s water can indicate a lot about the water’s quality and is important for the overall condition necessary for aquatic life, like flies, insects, plants & and assorted species of fish like Trout & Bass and Salmon, replenished and as healthy as possible with the right amount of oxygen. How much Oxygen do fish need?
The amount of Dissolved Oxygen (DO) needed varies for fish species. Bottom feeders-warm water fish (Catfish, Carp) need less oxygen (1-6 mg/L), shallow cold-water fish (Trout & Salmon) need higher levels (4-15 mg/L) some fish adapt to lower levels, Bluegill, Largemouth Bass, Perch for a short time.
Introduction:
Dissolved Oxygen or (DO) in a stream or any body of water may vary from 0 mg/l to 18 mg/l. In an aquatic environment, oxygen primarily comes from two main sources:
- Atmospheric Exchange: Oxygen enters water through diffusion at the water’s surface, where it comes into contact with the air. This exchange occurs due to the difference in oxygen concentration between the atmosphere and the water.
- Photosynthesis: Aquatic plants, such as algae and submerged vegetation, produce oxygen through photosynthesis. During photosynthesis, these plants use sunlight to convert carbon dioxide and water into oxygen and glucose, releasing oxygen as a byproduct into the water. This process contributes significantly to the oxygen levels in freshwater and marine environments.
How Much Oxygen Do Fish Need
Fish breathe with their gills, and they need a constant supply of oxygen. Gills sit under the operculum. This is called the gill slit. Many fish have four pairs of gills, while sharks may have up to seven. Even though fish can live their lives underwater, they still need oxygen to “breathe”. Instead of breathing air, fish must get their oxygen from the water.
This process requires large volumes of water to pass through absorption surfaces to get enough oxygen into their bodies using their mouths and gills. These body parts work like a pump to keep water moving over the gas absorption surfaces of the gills.
Just like in humans, the Carbon Dioxide in the fish is expelled with the water it takes in as the oxygen is pumped through their body in the water. Because there is normally more oxygen in the water than the gills of the fish, the oxygen from the water diffuses into the fish. The oxygen can then attach to hemoglobin, a protein in red blood cells that distributes oxygen throughout the body.
What is Dissolved Oxygen in Water
Dissolved oxygen (DO) measures how much oxygen is dissolved in the water – the amount of oxygen available to living aquatic organisms. The amount of dissolved oxygen in a stream or lake can tell us a lot about its water quality.
Dissolved water is water that is dissolved in a solution. As salt or sugar molecules dissolve in water, Oxygen molecules dissolve in water. Oxygen molecules can come from the surface of the body of water where the water is in constant contact with the water or through photosynthesis from living aquatic plant life within the body of the water that uses photosynthesis and releases oxygen as a byproduct. That Oxygen is released and slowly dissolves in the water.
Aquatic species like bacteria and fish depend on oxygen that’s dissolved in solution to live. There is always some Dissolved Oxygen underwater, but the amount can depend on some variables like temperature and salinity, among other organic materials that use up the dissolved oxygen in the water.
Some fish species can live with less, just like some fish species need more oxygen to live comfortably in their environment. Fish like Salmon adapt to cold water because colder water can hold more oxygen in dilution.
Factors Affecting Dissolved Oxygen
- Reduced DO levels in stream water may be because the water is too warm. The increased molecular activity of the warm water pushes the oxygen molecules out of the spaces between
 the moving water molecules.
the moving water molecules. - Decreased DO levels may also be indicative of too many bacteria and an excess amount of biological oxygen demand – BOD normally due to untreated sewage, partially treated sewage, and organic discharges, which use up all available dissolved oxygen in the receiving stream that can be seen as algae blooms.
- The third reason for decreased DO may be fertilizer runoff from farm fields and lawns. The same fertilizer, which was meant to make land plants grow better, now makes the aquatic plants do the same. If the weather becomes cloudy for several days, respiring plants will use much of the DO while failing to photosynthesize. When the increased numbers of aquatic plants eventually die, they support increasing amounts of bacteria that use large amounts of dissolved oxygen.
- The number of dissolved solids or suspended solids that can reduce DO levels in the water will affect the amount of oxygen that is dissolved in the water. So the clearer the water, the more DO can be dissolved in the water.
How much oxygen do fish need depends on these factors can result in fish and other aquatic life in the ecosystem dying because there won’t be enough DO or Dissolved Oxygen in the water to sustain life. This upsets the fish’s tolerance and the ecosystem’s Natural Balance which is heavily dependent on Oxygen for the ecosystem’s life.
Some kinds of fluctuations can affect the balance of nature, including the food source of the fish that live in the ecosystem. Depletion in DO can cause major shifts in the kinds of aquatic organisms found in water bodies.
Species that cannot tolerate low levels of DO – mayfly nymphs, stonefly nymphs, and beetle larvae – will be replaced by a few kinds of pollution-tolerant organisms, such as worms and fly larvae. Factors affecting the DO of the stream will end up affecting the fish that inhabit the stream eventually.
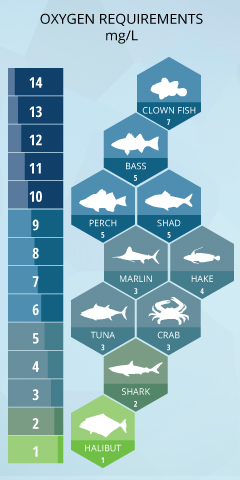
The amount of dissolved oxygen needed for aquatic species varies from creature to creature. Bottom feeders, crabs, oysters, and worms need minimal amounts of oxygen (1-6 mg/L), deep dwellers like Halibut and Shark need less, while more shallow water fish like Perch, Salmon, and Trout need higher levels (4-15 mg/L).
How Does Water Temperature Affect Dissolved Oxygen
 Although water molecules contain an oxygen atom H2O, this oxygen is not what is needed by aquatic organisms living in natural waters. Only a small amount of oxygen, up to about ten molecules of oxygen per million of water, is actually what is dissolved in water. Oxygen enters a river or a stream mainly from the atmosphere and, in areas where groundwater discharge into streams is a large portion of streamflow.
Although water molecules contain an oxygen atom H2O, this oxygen is not what is needed by aquatic organisms living in natural waters. Only a small amount of oxygen, up to about ten molecules of oxygen per million of water, is actually what is dissolved in water. Oxygen enters a river or a stream mainly from the atmosphere and, in areas where groundwater discharge into streams is a large portion of streamflow.
This dissolved oxygen is breathed by fish and zooplankton and is needed by them to survive. Fast-moving streams and creeks can pick up oxygen splashing over rocks from the current. This movement adds more healthy oxygen to dissolve in the water.
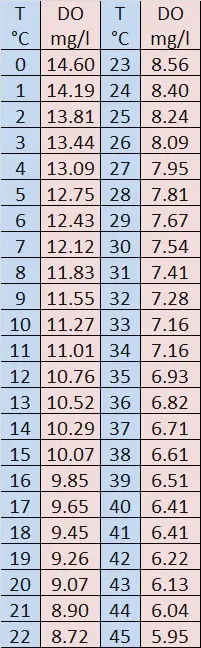
The cooler or colder the stream’s water temperature is, the more oxygen it can absorb. As the water movement slows and warms, becoming stagnant, the water contains less oxygen. These water Temperatures have a direct effect on how much Dissolved Oxygen the H2O can saturate or hold in solution.
As the seasons change and temperatures rise, the water dissolved oxygen becomes less the surface temperature can rise to deplete the levels of oxygen. Excess organic material in lakes and rivers can cause eutrophic conditions, which is an oxygen-deficient situation that can cause a water body to “die.” Aquatic life, including fish, can have a hard time in stagnant water that has a lot of rotting organic material in it, especially in summer temperatures.
Too many nutrients needed by green plants and cyanobacteria to grow or bloom will end up hurting the environment once they die and start to decay, which will use up all the oxygen that is available for a normal balance.
Conditions may become especially serious during a period of hot, calm weather, resulting in the death of many fish. You may have heard about summertime fish kills in local lakes that likely result from this problem. Nutrients like nitrogen and phosphorus can be created by pollution or natural decay that can result in algae blooms, especially when warmer temperatures arrive.
This occurrence will deplete the Dissolved Oxygen at a faster rate than it can be replaced. These conditions, called Eutrophication, can cause fish kills that eventually cause more oxygen depletion. This happens a lot at effluents of Wastewater Treatment Plants as a result of the overabundance of nutrients that are a byproduct of sewage which will cause algae blooms, mostly green but sometimes red in the case of Florida that suffers from the dreaded “Red Tide”
What is the Best Water Temperature for Trout Activity?
Trout are cold-blooded fish that prefer to live & feed in colder H2O around 53°F which holds more dissolved oxygen but as H2O warms, oxygen begins to dissipate, and trout become less active and will move up & down in the water column or to a different location to find colder temperatures …………………………………………………………………… Read more
How Much Oxygen Do Fish Need

Fish require varying amounts of oxygen depending on their species, size, activity level, and environmental conditions. Generally, fish need dissolved oxygen in water to survive, as they extract oxygen through their gills.
Adequate oxygen levels in water are crucial for fish respiration, metabolism, and overall health. Oxygen levels below certain thresholds can lead to stress, reduced growth rates, and even death in fish populations. Oxygen requirements can differ significantly between species, with some being more tolerant of low-oxygen conditions than others.
The amount of Dissolved Oxygen needed varies from creature to creature Trout is right on top for freshwater fish. Bottom feeders, crabs, oysters, and worms need minimal amounts of oxygen (1-6 mg/L), while shallow-water fish need higher levels of 4-15 mg/L. Microbes such as bacteria and fungi also require dissolved oxygen.
DO is necessary for many forms of life, including fish, invertebrates, bacteria, and plants. These organisms use oxygen in respiration, similar to organisms on land.
Fish and crustaceans obtain oxygen for respiration through their gills, while plant life and phytoplankton require dissolved oxygen for respiration when there is no light for photosynthesis.
You can see that cold-water fish that are normally found in the Alaskan streams that run off mountains fed by snowcaps, like Trout and Salmon, are found in freshwater with higher DO Mg/L, and Northern Alaskan cold-water Halibut and Crab that are in the North Atlantic are found in deep cold water with a little less Dissolved Oxygen content.
DO levels rise from morning through the afternoon as a result of photosynthesis, reaching a peak in the late afternoon. Photosynthesis stops at night, but plants and animals continue to respire and consume oxygen. As a result, DO levels fall to a low point just before dawn?
How Do You Tell the Difference Between Trout?
3 primary species of trout:
Brown Trout-brownish-yellow color-scattered black, red,& orange spots on their sides-12″ long or less
Brook Trout-(Speckled trout) brown with reddish fins & white tips-8″ long or less
Rainbow Trout-dark back & white belly-pink stripe down middle-up to 12″ long are common .……………………………………………………………………………. Read more
Low Dissolved Oxygen Consumption
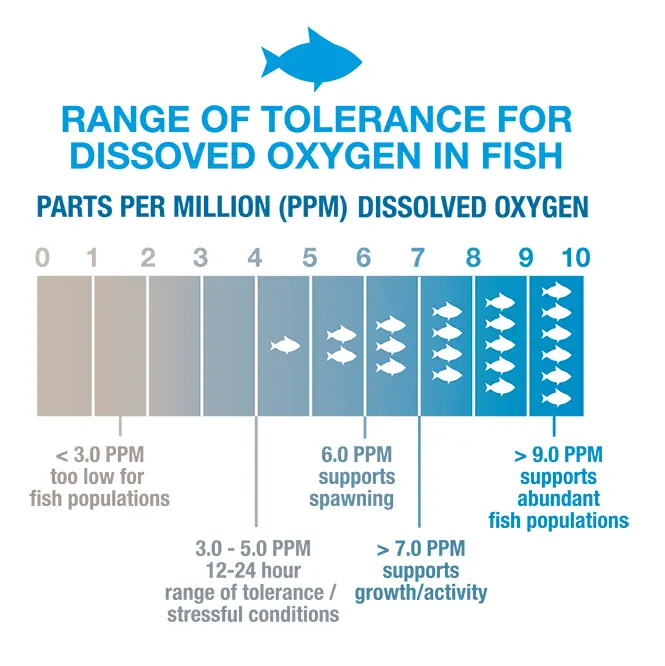
Dissolved oxygen levels may dip below 4 mg/l in such waters – the minimum amount needed to sustain warm water fish like bluegill, bass, and pike. Under EPA laws in most states, 4.0 mg/l is a constant parameter for receiving streams from Wastewater Effluents to ensure the balance of the river or stream is always met. Not only for fish but for the mayflies, caddisflies, stoneflies, and all aquatic life that populates the ecosystem.
A Trout needs five to six times more DO when the water temperature is 24 degrees C (75 degrees F) as compared to when the water temperature is 4 degrees C (41 degrees F). The increased DO is needed to support an increase in metabolic rates – a phenomenon shared by other cold-blooded aquatic animals.
The generally accepted minimum amount of DO that will support a large population of various fish is from 4 to 5 mg/l. With 5mg/l being preferable for a fish’s overall health. When the DO drops below 3 mg/l, even the hardy fish die.
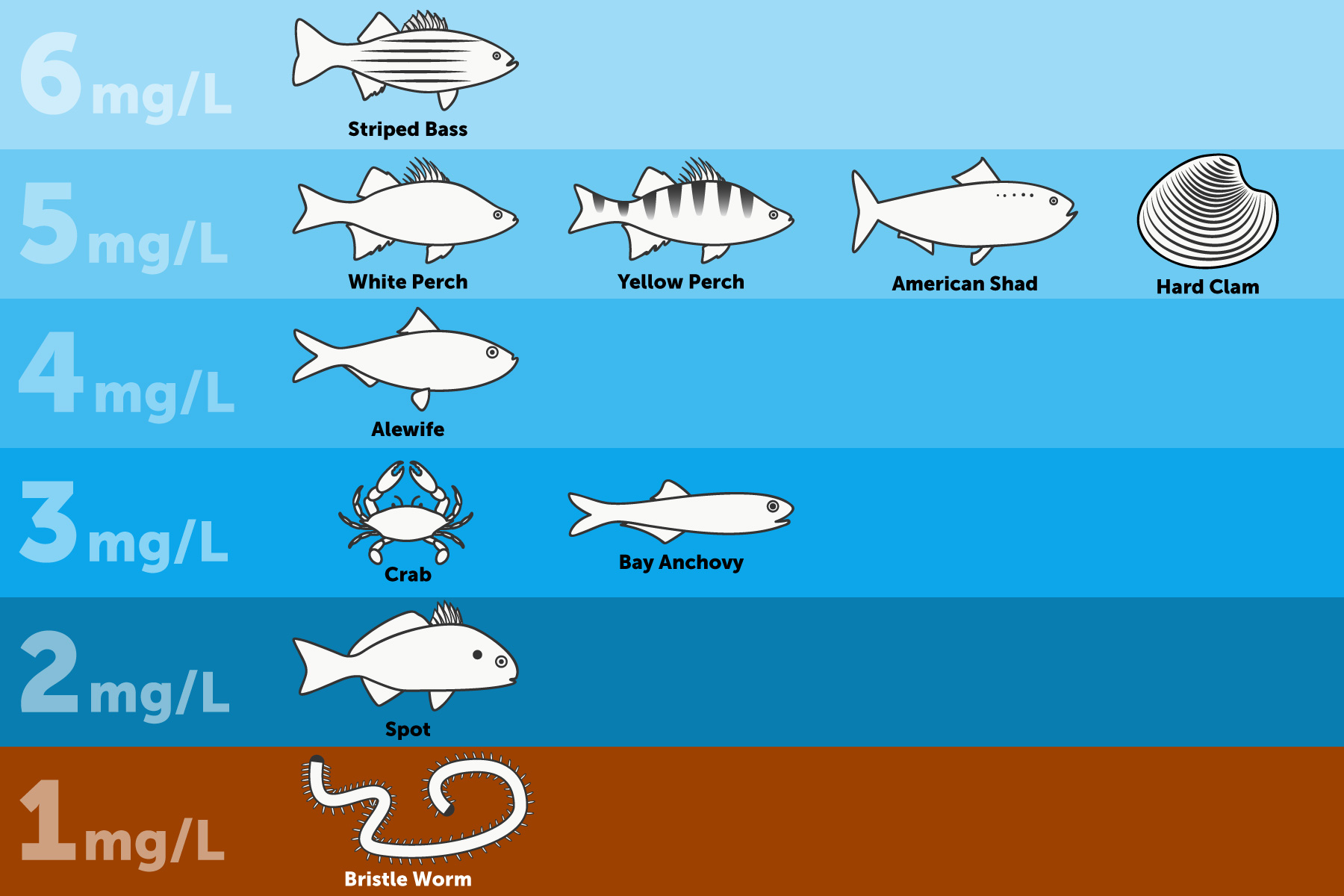
Bluegill, Largemouth Bass, White Perch, and Yellow Perch are considered warm-water fish and depend on dissolved oxygen levels above 5 mg/L. They will avoid areas where DO levels are below 3 mg/L but generally do not begin to suffer fatalities due to oxygen depletion until levels fall below 2 mg/L. The mean DO levels should remain near 5.5 mg/L for optimum growth and survival
Northern Pike – 6.0 mg/L
Black Bass – 5.5 mg/L
Common Sunfish – 4.2 mg/L
Yellow Perch – 4.2 mg/L
Black Bullhead – 3.3 mg/L
Trout and Salmon generally attempt to avoid areas where dissolved oxygen is less than 5 mg/L and will begin to die if exposed to DO levels less than 3 mg/L for more than a couple of days. Other species might stay for a while and become tolerant for short periods before moving on.
Why are Oxygen Depletion Events Most Troublesome in the Summer
How To Measure Dissolved Oxygen In Water Column
 The more oxygen in a body of water, the more easily aquatic organisms can survive and flourish, making the environment healthy and adaptable for fish to populate and for food sources to be available.
The more oxygen in a body of water, the more easily aquatic organisms can survive and flourish, making the environment healthy and adaptable for fish to populate and for food sources to be available.
State Fisheries use high standards for Dissolved Oxygen. In Coldwater fisheries, the parameter should never go below 4mg/l, and for warm-water fisheries. They also use a 30-day average that should never go below 6.5 mg/l for Coldwater fish and 5.5 mg/l for Warmwater fish.
Having worked for more than 30 years in wastewater treatment DO or dissolved oxygen was an important parameter for discharge to streams or rivers and was set at 4 to 5 mg/l.
There are a couple of ways to measure DO in the field and a lab setting DO Chlorimetric Kit, which is found on Amazon that will measure a known color comparison like you would in a swimming pool or a Winkler method that has been used in Water testing Labs for many years.
For the serious Fisherman toolbox, a measurement for Dissolved Oxygen will show you in the field how healthy the water you are fishing in is. Now Portable DO Meters are available and very reliable and easy to celebrate and operate. Fish need oxygen to survive low oxygen concentrations and low dissolved oxygen levels
- pH, Conductivity or Salinity or TDS, Dissolved oxygen & Temperature
- Handheld fashion housing with IP67 waterproof design
- Auto temperature compensation
- Auto-ranging for conductivity measurement
Check these guys out! and take your Tacklebox to another level. If you buy one, you should get one that is rechargeable, waterproof, and comes with a probe that you can use from standing above.
Conclusion:
The amount of oxygen dissolved in a stream or lake’s water can indicate a lot about the water’s quality and is important for the overall condition necessary for aquatic life, like flies, insects, plants & and assorted species of fish like Trout & Bass and Salmon, replenished and as healthy as possible with the right amount of oxygen. How much Oxygen do fish need?
The amount of Dissolved Oxygen (DO) needed varies for fish species. Bottom feeders-warm water fish (Catfish, Carp) need less oxygen (1-6 mg/L), shallow cold-water fish (Trout & Salmon) need higher levels (4-15 mg/L) some fish adapt to lower levels, Bluegill, Largemouth Bass, Perch for a short time.
Dissolved Oxygen or (DO) in a stream or any body of water may vary from 0 mg/l to 18 mg/l. Readings above 18 mg/l are physically impossible. Dissolved oxygen gets into the water by diffusion from the atmosphere, aeration of the water as it tumbles over falls and rapids, and as a waste product of photosynthesis.
What is the Best Rig for Rainbow Trout?
3 Main types of Rainbow Trout Rigs:
Rigs that present bait suspended underneath a float
Rigs that present the bait at/near the bottom
Rigs that allow you to cast & retrieve artificial lures like the Rooster Tail based on color, baitfish, or insect type the best …………………………………………………………. Read more

References: How Do Fish Breathe? The Science Behind Gills
USGS–Dissolved Oxygen and Water
FAQ’s
How do fish breathe underwater? Fish breathe underwater using gills, specialized organs that extract oxygen from the water. As water passes over the gills, oxygen is absorbed into the bloodstream while carbon dioxide is released back into the surrounding water

